Worksheet 1 Equilibrium Constants Answer Key Conceptual Answers 1 The magnitude of the equilibrium constant for a reaction depends on the form in which the chemical reaction is written For example writing a chemical reaction in different but chemically equivalent forms causes the magnitude of the equilibrium constant to be different but can be related by comparing their respective
Worksheets Equilibrium Constant Worksheet 1 Equilibrium Constant Worksheet 1 Key Robert E Belford University of Arkansas Little Rock Department of Chemistry The breadth depth and veracity of this work is the responsibility of Robert E Belford rebelford ualr edu You should contact him if you have any concerns Q8 8 Complete the following charts by writing left right or none for equilibrium shift and decreases increases or remains the same for the concentrations of reactants and products and for the value of K e q Remember that pure solids and liquids do not affect equilibrium values N 2 g 3 H 2 g 2 N H 3 g with H 92 k J
Worksheet 1 Equilibrium Constants Answer Key

Worksheet 1 Equilibrium Constants Answer Key
https://i2.wp.com/s3.studylib.net/store/data/007750003_2-152a8ba33a2fa05c8df8b56320adaafc.png

Solved WORKSHEET CHEMICAL EQUIUBRIUM FOR ALL EQUILIBRIUM Chegg
https://media.cheggcdn.com/study/460/4600cb76-8b30-4caf-ad38-9edc0685fb9a/image.png
CHEMISTRY 112
http://www.oneonta.edu/faculty/viningwj/Chem112/Equil_Handout2.jpg
2 A mixture of 9 22 moles of A 10 11 moles of B and 27 83 moles of C is placed in a one liter container at a certain temperature The reaction is allowed to reach equilibrium At equilibrium the number of moles of B is 18 32 Calculate the equilibrium constant for the reaction A g 2 B g 3 C g Ans 0 832 Write the equilibrium expression for the reaction and calculate the value of the equilibrium constant for each experiment K H2 CO 2 H2O CO Expt 1 K 0 774 Expt 2 K 0 774 Expt 3 K 0 773 4 In the following reaction at 448 the equilibrium concentrations are HI 0 0040M
CHEMICAL EQUILIBRIUM ICE METHOD Chemical equilibrium occurs when opposing reactions are proceeding at equal rates The rate at which the products are formed from the reactants equals the rate at which the reactants are formed from the products As a result concentrations cease to change making the reaction appear to be stopped CHM152 Equilibrium Worksheet Key 1 Equilibrium Worksheet Key 1 2NH 3 g N 2 g 3H 2 g At 500 K the following concentrations were measured N 2 3 0 x 10 2 M H 2 Check answer by plugging concentrations into K c 7 Calculate the equilibrium concentrations of all species if 3 000 moles of H 2 and 6 000 moles
More picture related to Worksheet 1 Equilibrium Constants Answer Key

Solved Be Sure To Answer All Parts The Following Chegg
https://media.cheggcdn.com/study/06e/06e1bee9-17ad-42e1-b018-d8b27455714e/image.png
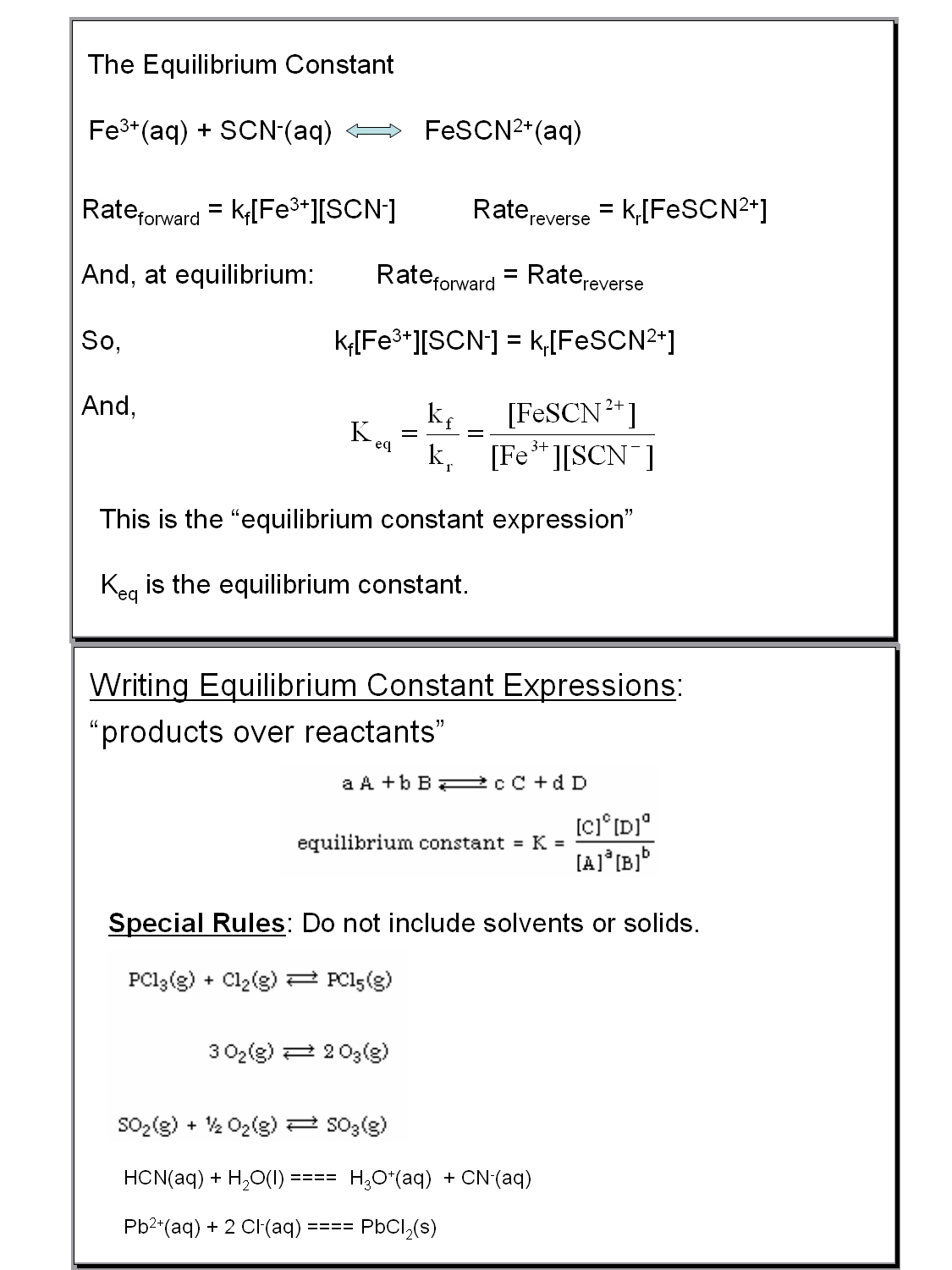
Answer Key For Chemical Equilibria 1 Equilibrium Constant
https://s3.studylib.net/store/data/006834448_2-94cf5db6d9768736bbdc397ee3d32911.png

03 Equilibrium Constant Worksheet 2 Answers EQUILIBRIUM CONSTANT
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/8371c29c205a2d505bbc30bec7be8b76/thumb_1200_1553.png
About this unit This unit explores the how and why of chemical equilibrium Learn about reversible reactions the equilibrium constant Le Ch telier s principle solubility equilibria and more Practice what you ve learned and study for the AP Chemistry exam with more than 80 AP aligned questions The activation energy would decrease Increase Increase One mole of very cold colorless N2O4 g is placed into a 1 0L glass container of room temperature The reaction N2O4 g 2 N02 g H 58KJ colorless brown proceeds to equilibrium The concentration of each gas is measured as a function of time
At equilibrium the concentration of HBr is 0 L Assuming H 2 and Br 2 are present in equal amounts calculate the concentration of H 2 at equilibrium H 2 0 mol L Analysis of the following equilibrium reaction at 900 C provides the concentrations listed below 2 g CO g H 2 g COH O 2 g Writing equilibrium constant expressions Google Classroom You might need Periodic table What is the equilibrium constant expression K c for the following balanced equation PbCl 2 s Pb 2 a q 2 Cl a q
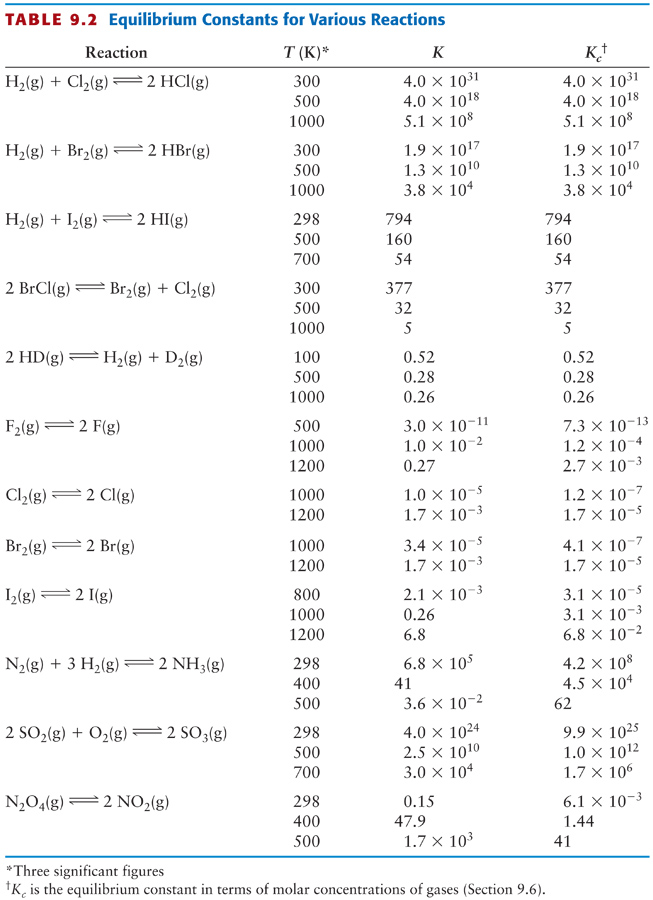
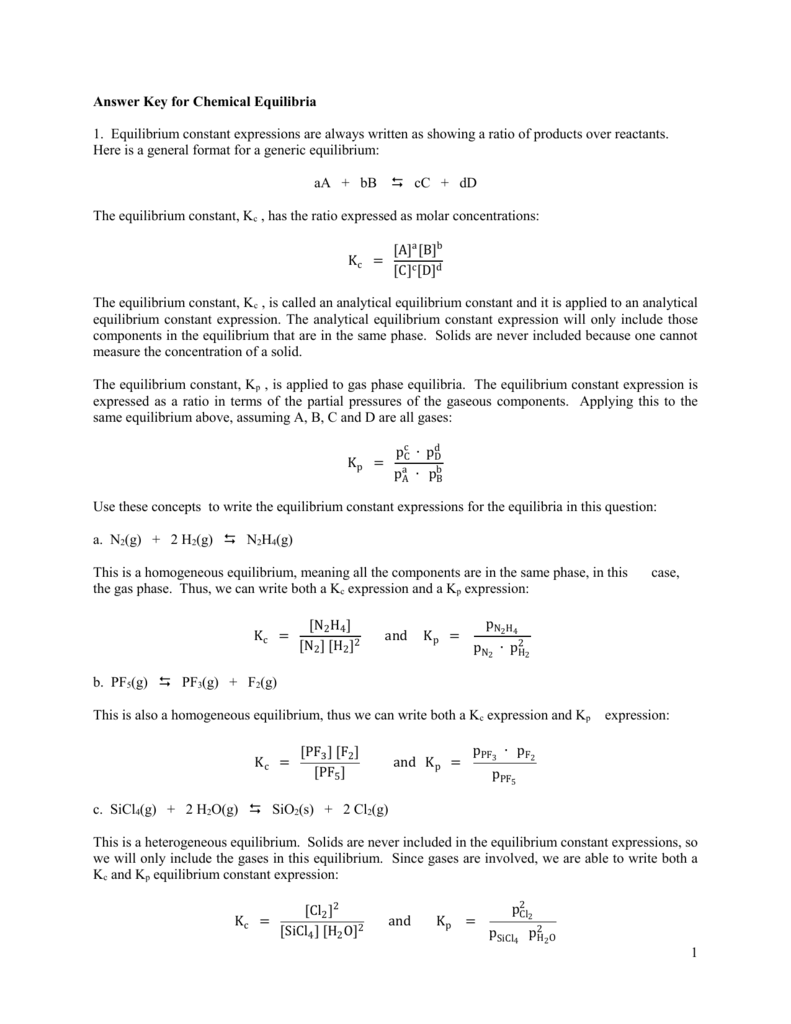
Untitled Document people chem umass edu
http://people.chem.umass.edu/botch/Chem122S08/Chapters/Ch9/EquilibriumConstant/TableRxnsK.jpg
Solved Experiments Have Shown That The Equilibrium Constant Kp For
https://www.coursehero.com/qa/attachment/11743575/
Worksheet 1 Equilibrium Constants Answer Key - Equilibrium Constant Worksheet 1 Write the equilibrium expression for the following reactions The Keq for this reaction at 25 is 1 02 At equilibrium the concentration of HBr is 0 50mol L Assuming H2 and Br2 are present in equal amounts calculate the concentration of H2 at equilibrium Analysis of the following equilibrium reaction at