Which Option Will Not Increase The Rate Of Reaction Nov 21 2024 nbsp 0183 32 The option that does NOT increase the rate of a reaction is C Decreasing the surface area This is because a decrease in surface area limits the contact between reactants
Feb 2 2021 nbsp 0183 32 The situation that does NOT increase the reaction rate of most chemical reactions is option D Decreasing the concentrations of the substances in the reaction This is because Nov 16 2023 nbsp 0183 32 The option that would NOT increase the rate of a chemical reaction is C Increasing the activation energy barrier This is because a higher activation energy makes it
Which Option Will Not Increase The Rate Of Reaction
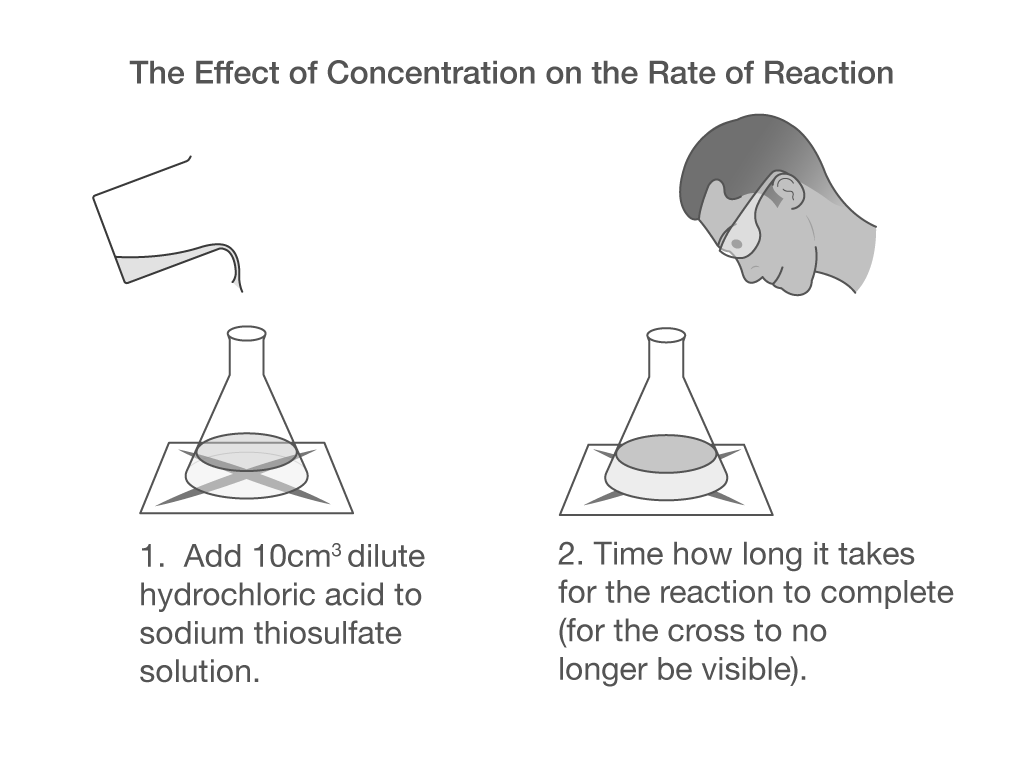
Which Option Will Not Increase The Rate Of Reaction
https://www.lbq.org/filestore/questionsupplement/e5bcb411-cf13-4882-bad5-58f2f3147845.png

Investigating Effect Of PH On Enzyme Amylase Activity GCSE Biology
https://i.ytimg.com/vi/id-MSKnNqrM/maxresdefault.jpg

Amazon Affiliate AtomicBrom
https://static.wixstatic.com/media/565451_6a6ade554abd4af599f51a61fc31fb24~mv2.png/v1/fit/w_2500,h_1330,al_c/565451_6a6ade554abd4af599f51a61fc31fb24~mv2.png
May 12 2024 nbsp 0183 32 The presence of an enzyme Enzymes are biological catalysts that speed up reactions They provide an alternative pathway for the reaction with a lower activation energy The rate law of the overall reaction A B C is rate k A 2 Which of the following will not increase the rate of the reaction Question 4 options
Question Which of the following will NOT increase the rate of a reaction 1 Addition of more reactants to the reaction mixture II Maintaining constant temperature of the reaction mixture The addition of a homogeneous catalyst does not change the activation energy of a given reaction Rate constants are temperature dependent A catalyst lowers the activation energy
More picture related to Which Option Will Not Increase The Rate Of Reaction

Publications Petrolloyd Research Group
https://petrolloydresearchgroup.com/wp-content/uploads/2022/10/Petrolloyd-Research-Group.png
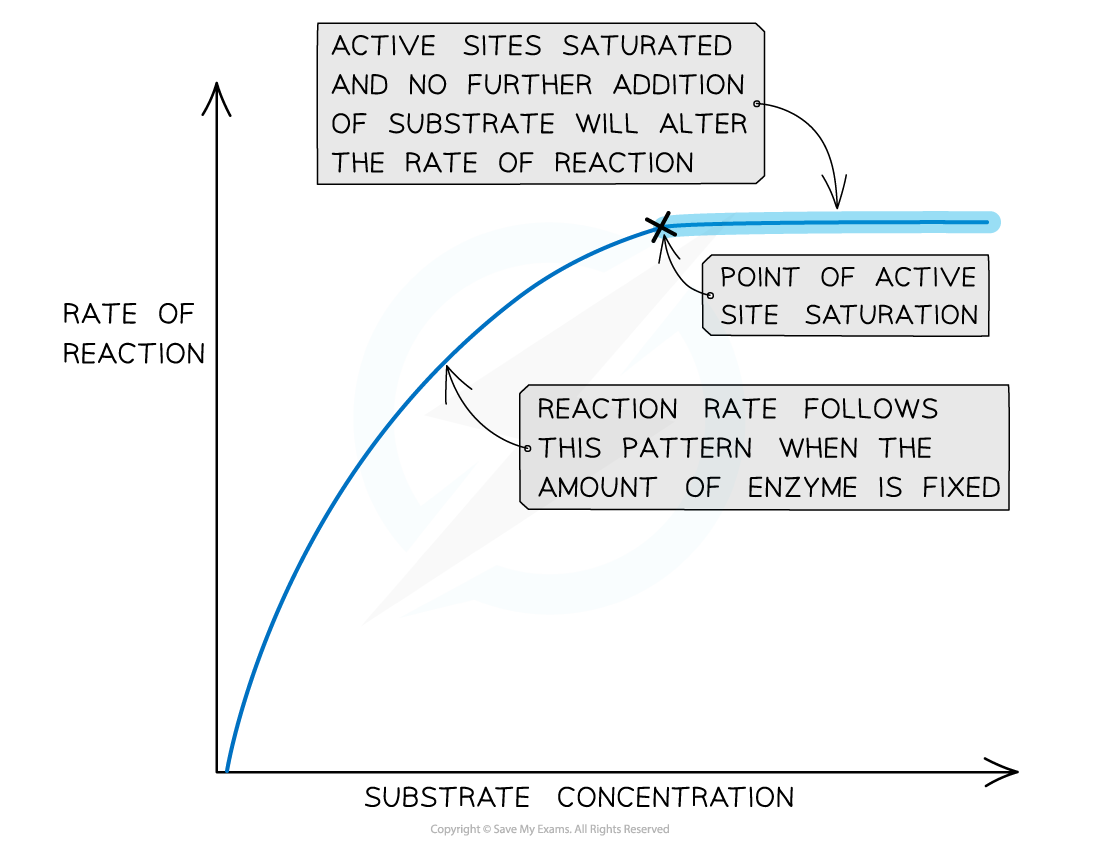
CIE A Level Biology 3 2 4 Rate Substrate Concentration
https://oss.linstitute.net/wechatimg/2022/08/The-effect-of-substrate-concentration-on-an-enzyme-catalysed-reaction-3.png

Campus Details Birdville High School
https://www.birdvilleschools.net/cms/lib/TX01000797/Centricity/Domain/10018/PropBLogo.png
Which of the following changes will not increase the rate of a chemical reaction A Increasing the concentration of reactants B Increasing the surface area of a solid reactant C Decreasing Our expert help has broken down your problem into an easy to learn solution you can count on There are 2 steps to solve this one The rate of reaction is th Not the question you re looking
2 Heating the beaker Option A will increase the rate of reaction due to the increase in temperature 3 Adding more limestone Option B will increase the rate of reaction as there will Study with Quizlet and memorize flashcards containing terms like Which of the following factors affect the rate of a chemical reaction Choose all that apply Addition of a catalyst to the

Logo Redraw Service DTF Print Hub Australia
https://cdn.shopify.com/s/files/1/0638/9193/1380/files/DTF_Print_Hub_Logo_Final_WEB_HEADER_dbab9c2d-d624-4869-a69b-fb3647009b20.png?v=1669985988

What Of The Following Is An Accurate Statement Brainly
https://us-static.z-dn.net/files/d8b/346858eb58c11be1f0c6efc8ae72a0c0.png
Which Option Will Not Increase The Rate Of Reaction - Dec 21 2016 nbsp 0183 32 The option that would not increase the rate of most reactions is B Lowering the concentration of the reactants as this would decrease the frequency of collisions between the