What Ray Has Two Protons And Two Neutrons Particle alpha is a helium nucleus two protons and two neutrons It has a large mass compared to other ionising radiations and a strong positive charge particle beta is a fast
Mar 19 2020 nbsp 0183 32 There are four major types of radiation alpha beta neutrons and electromagnetic waves such as gamma rays They differ in mass energy and how deeply they penetrate people and objects The first is an alpha particle These particles consist of two protons and two neutrons and are the heaviest type of radiation particle May 8 2022 nbsp 0183 32 An alpha particle is a particle consisting of two protons two neutrons and no electrons Essentially it is a helium 4 nucleus Other names for alpha particles are alpha rays or alpha radiation The symbol for an alpha particle is 2 He 2 or 4 2 He 2
What Ray Has Two Protons And Two Neutrons

What Ray Has Two Protons And Two Neutrons
https://media.nagwa.com/404194847014/en/thumbnail_l.jpeg
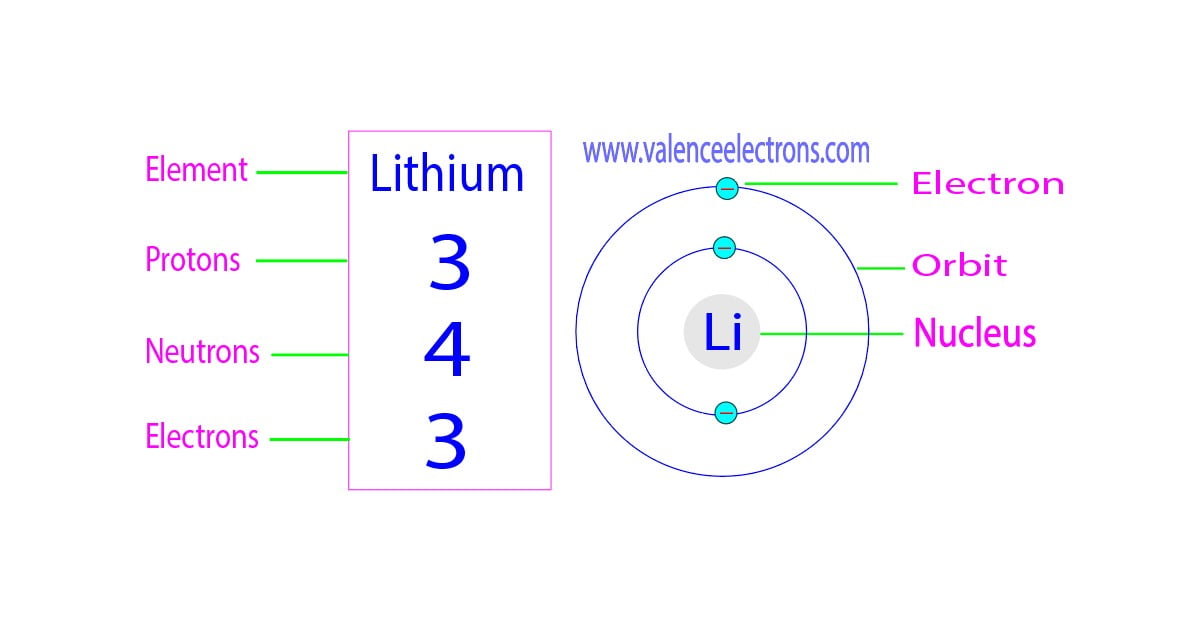
How Many Protons Neutrons And Electrons Does Lithium Have
https://valenceelectrons.com/wp-content/uploads/2022/06/Lithium-protons-neutrons-electrons.jpg
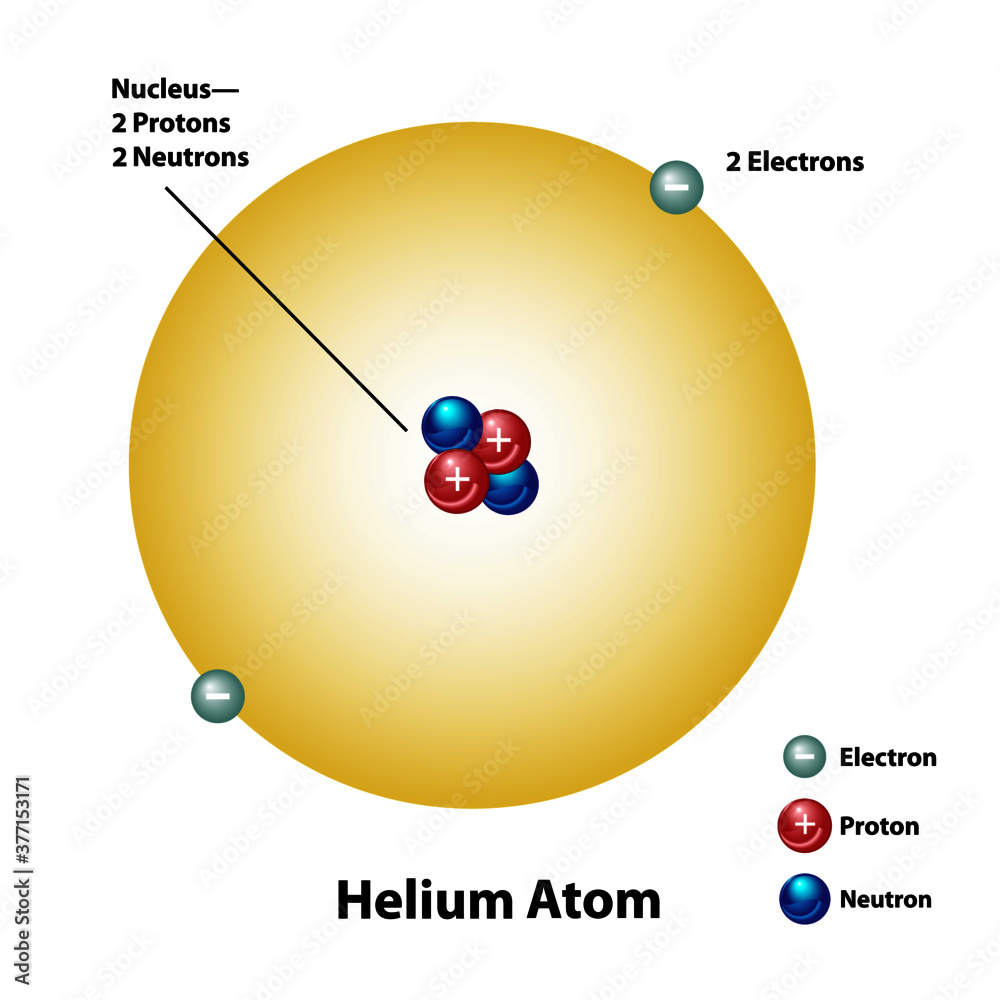
Helium Molecular Element Diagram Showing Mass Protons Electrons
https://as2.ftcdn.net/v2/jpg/03/77/15/31/1000_F_377153171_RPztLk6nlIqTSqPTqXvX3BJqKwB5YTOp.jpg
The mass number of an atom is equal to the sum of protons and neutrons in the nucleus A helium atom has 2 protons and 2 neutrons What is the mass number of this atom How many neutrons are present in this radioisotope A 15 B 17 C 32 D 47 and more Study with Quizlet and memorize flashcards containing terms like An alpha particle is a high energy particle that contains A one proton and one neutron
Alpha radiation consists of alpha particles that are energetic nuclei of helium The production of alpha particles is termed alpha decay Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus Alpha particles are relatively large and carry a double positive charge If the nucleus is unstably large it will emit a package of two protons and two neutrons called an alpha particle An alpha particle is also a helium 4 nucleus so it is written as 4 2 He It
More picture related to What Ray Has Two Protons And Two Neutrons
No Matter The Size Of A Nuclear Party Some Protons And Neutrons Will
https://news.mit.edu/sites/default/files/download/202011/MIT-Universal-Cloud-01-press.jpg
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
Why Protons And Neutrons Stick Together In The Nucleus
https://www.thoughtco.com/thmb/_8_TLY7mSDbMSgg_r_Qjth3habI=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg
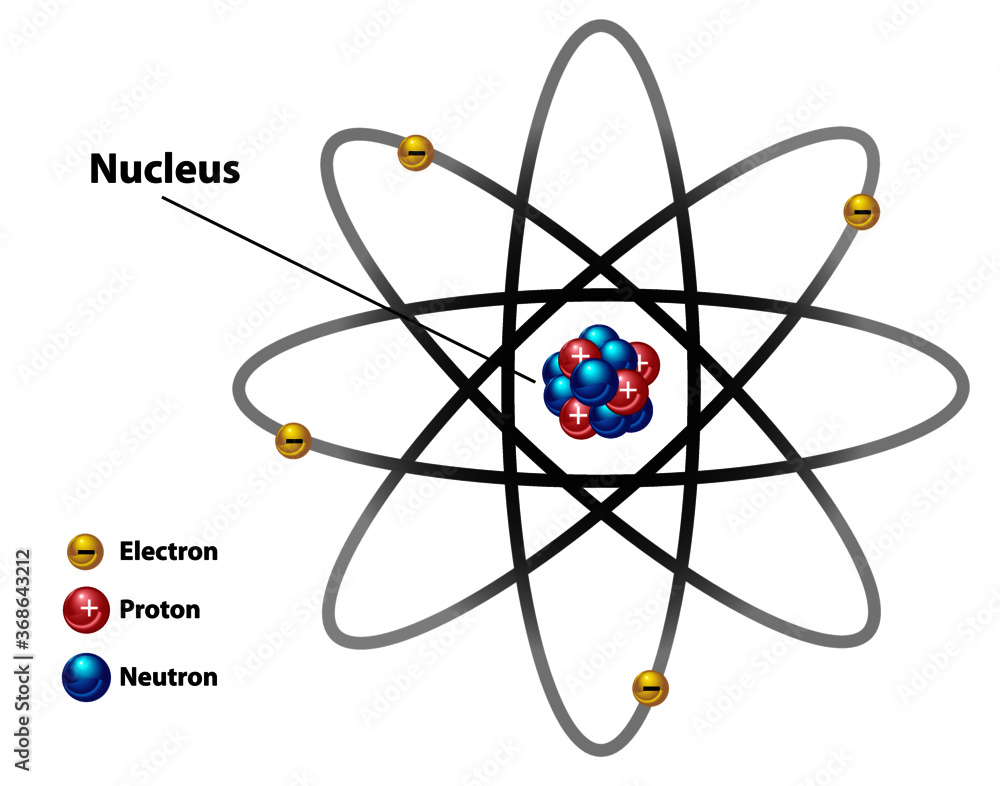
Atomic Nucleus Diagram Labeled With Electron Proton And Neutron
https://as1.ftcdn.net/v2/jpg/03/68/64/32/1000_F_368643212_ovdSJUrD6hM0crl4B03QzcQ55XlyowRS.jpg
If the nucleus has too few neutrons it will emit a package of two protons and two neutrons called an alpha particle Mar 7 2019 nbsp 0183 32 An alpha particle is a positively charged particle emitted by various radioactive materials during decomposition It consists of two neutrons and two protons
Dec 27 2023 nbsp 0183 32 Alpha rays consist of two protons and two neutrons and have two positive charges Beta Rays consist of electrons and have a negative charge while Gamma Rays consist of photons and are neutral In this article we will learn what are alpha beta and gamma rays along with their properties and comparison between them in tabular form Dec 4 2024 nbsp 0183 32 Alpha decay is a type of radioactive decay in which an unstable atomic nucleus releases an alpha particle to become more stable An alpha particle consists of two protons and two neutrons making it similar to the nucleus of a helium atom 4 He 2 with a 2 charge

Proton Neutron Electron Chart
https://physfox.s3.eu-west-2.amazonaws.com/!electricity/pne/table-pne.png
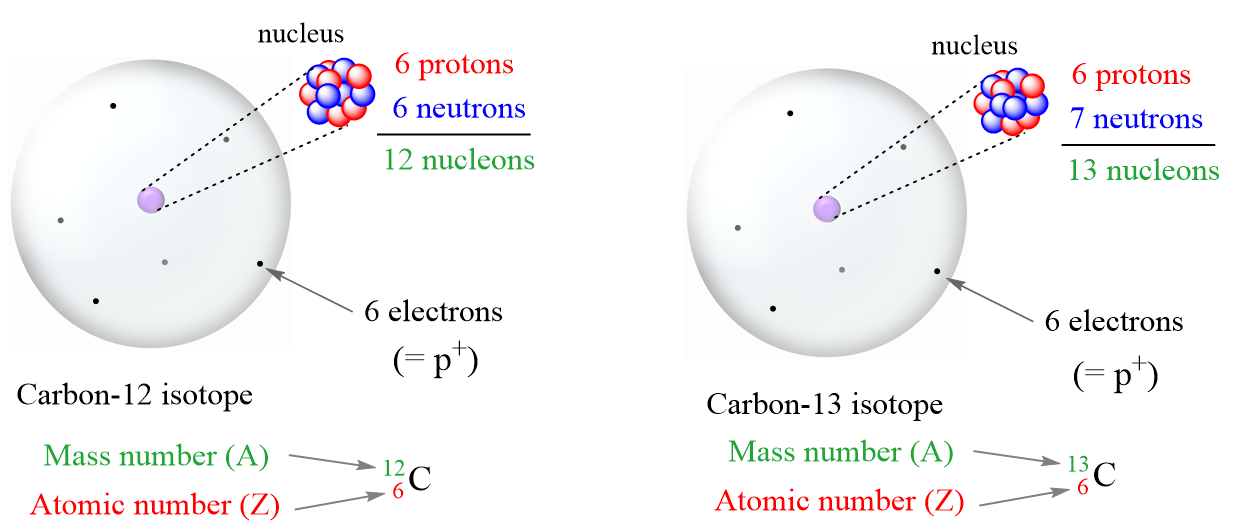
How To Calculate The Number Of Protons Neutrons And Electrons
https://general.chemistrysteps.com/wp-content/uploads/2022/03/Isotopes-protons-neutrons-electrons-and-mass-number.png
What Ray Has Two Protons And Two Neutrons - The mass number of an atom is equal to the sum of protons and neutrons in the nucleus A helium atom has 2 protons and 2 neutrons What is the mass number of this atom