What Is Rate Of Reaction Measured In Chemistry Feb 13 2023 nbsp 0183 32 Definition of Reaction Rate The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time The speed of a chemical reaction may be defined as the change in concentration of a substance divided by the time interval during which this
The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction It gives some insight into the time frame under which a reaction can be completed For example the reaction rate of the combustion of cellulose in fire is very high and the reaction is completed in less than a second Jul 12 2023 nbsp 0183 32 What follows is general guidance and examples of measuring the rates of a reaction Measuring time change is easy a stopwatch or any other time device is sufficient However determining the change in concentration of the reactants or products involves more complicated processes
What Is Rate Of Reaction Measured In Chemistry

What Is Rate Of Reaction Measured In Chemistry
https://i.ytimg.com/vi/LstMh4V2PiA/maxresdefault.jpg

Rate Of Reaction What Is Rate Of Reaction Example YouTube
https://i.ytimg.com/vi/7V0w4oajO1I/maxresdefault.jpg

GCSE Chemistry How To Calculate The Rate Of Reaction Measuring Rate
https://i.ytimg.com/vi/GCR5xeduq2o/maxresdefault.jpg
To measure reaction rates chemists initiate the reaction measure the concentration of the reactant or product at different times as the reaction progresses perhaps plot the concentration as a function of time on a graph and then calculate the change in the concentration per unit time Nov 20 2024 nbsp 0183 32 Reactions take place at different rates depending on the chemicals involved and the conditions Some are extremely slow e g rusting and others are extremely fast e g explosives The rate of reaction can be measured in two different ways How fast a reactant is used up How fast a product is made Three methods used to determine the rate of
What is reaction rate How to calculate it Learn its equation and unit What are the factors that affect the reaction rate Reaction rate in chemistry the speed at which a chemical reaction proceeds It is often expressed in terms of either the concentration amount per unit volume of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time
More picture related to What Is Rate Of Reaction Measured In Chemistry

Rate Of Reaction And Rate Law Chemistry Class 12 IIT JEE Main
https://i.ytimg.com/vi/3veVBN1zYCo/maxresdefault.jpg
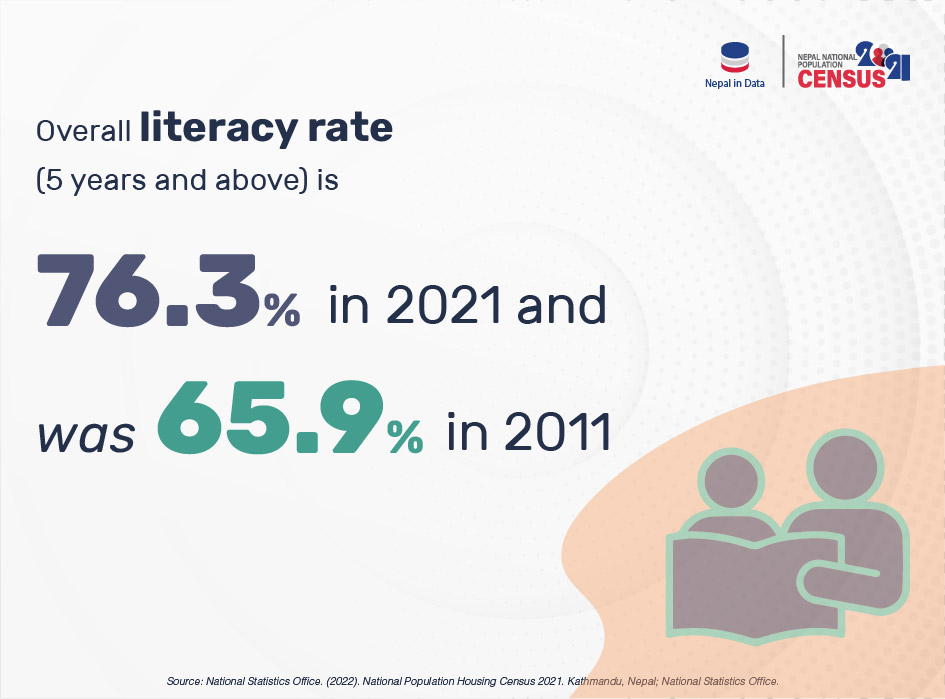
LITERACY RATE 2021 Infograph
https://nepalindata.com/media/insight/23._Literacy_Rate_Ca6dvDW.jpg

The Rate Equation A Level ChemistryStudent
https://www.chemistrystudent.com/images/A2Physical/kinetics/rateequation1blank.png
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time 1 What is a rate equation in chemistry A rate equation is a mathematical expression that describes the relationship between the rate of a chemical reaction and the concentrations of the reactants How is the rate of a chemical reaction measured
The rate of reaction is the change in the amount of a reactant or product per unit time Reaction rates are therefore determined by measuring the time dependence of some property that can be related to reactant or product amounts Two ways to measure the volume of a gas produced in a reaction The rate of reaction can be analysed by plotting a graph of mass or volume of product formed against time The graph shows this

Chemistry Formula For Rate Constant For The First Order Reaction
https://i.stack.imgur.com/3UhVw.jpg
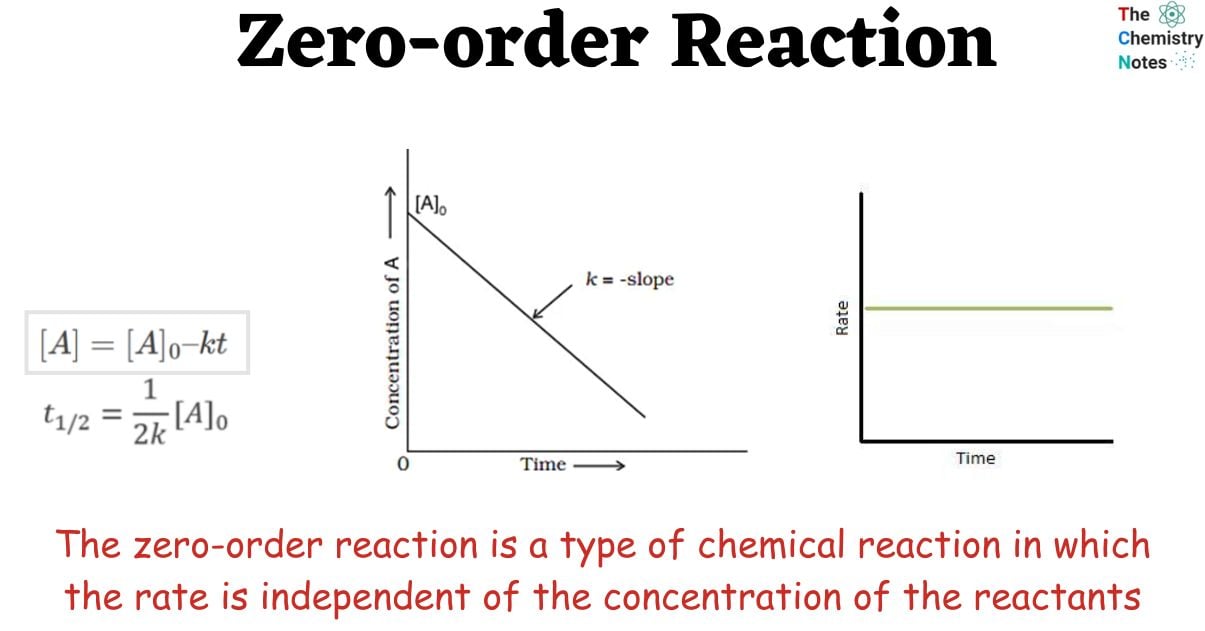
Zeroth Order
https://scienceinfo.com/wp-content/uploads/2023/06/Zero-order-Reaction.jpg
What Is Rate Of Reaction Measured In Chemistry - In a chemical reaction a substance converts into another substance under certain given conditions in a given time It is important to know the rate of a chemical reaction to completely understand the reaction Which are the slowest and the fastest reactions in the world