What Is Empirical And Molecular Formula Aug 22 2020 nbsp 0183 32 The empirical formula is the simplest whole number ratio of elements while the molecular formula is actual ratio of elements The molecular formula is a multiple of the empirical formula The empirical and molecular formulas are two types of chemical formulas that tell you the ratios or proportions of elements in a compound The empirical or
The empirical formula of a compound gives the simplest ratio of the number of different atoms present whereas the molecular formula gives the actual number of each different atom present in a molecule You can work out the molecular formula from the empirical formula if you know the relative mass formula M r of the compound Add up the atomic masses of the atoms in the empirical
What Is Empirical And Molecular Formula
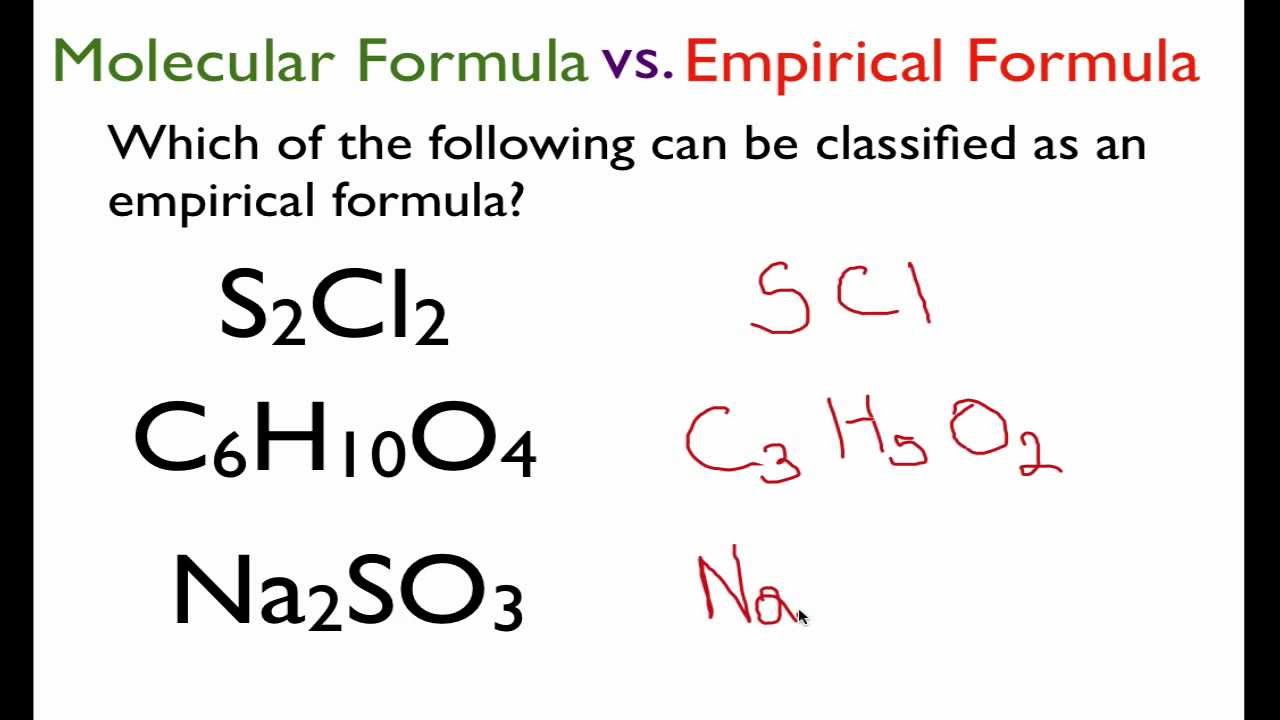
What Is Empirical And Molecular Formula
https://i.ytimg.com/vi/0Ikv_o7gcAE/maxresdefault.jpg
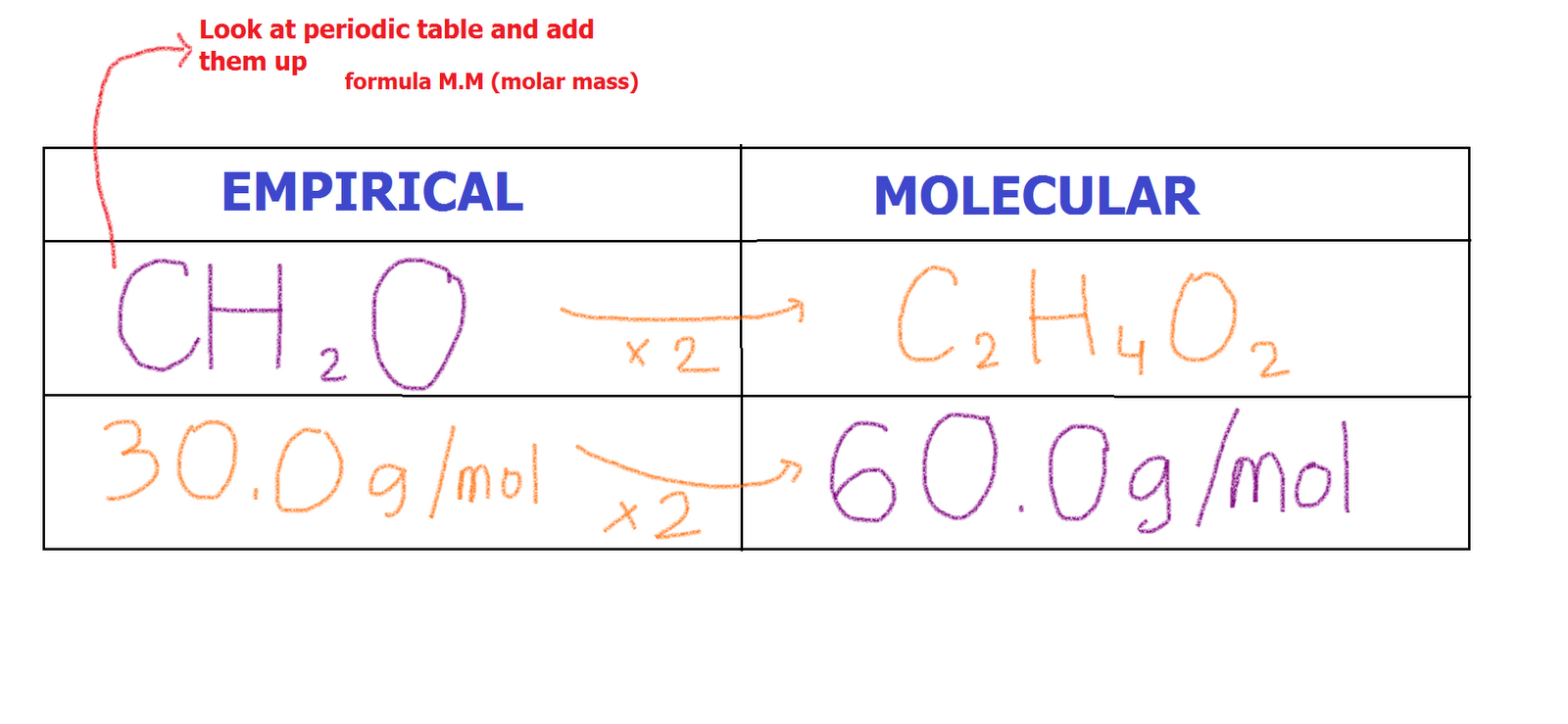
Alisia Tobler Formula For Finding Volume Chemistry
https://3.bp.blogspot.com/_z9emnNOdBIE/TT5cH5fDryI/AAAAAAAAA2I/qAcgVzsqxb8/s1600/molecular+example.png
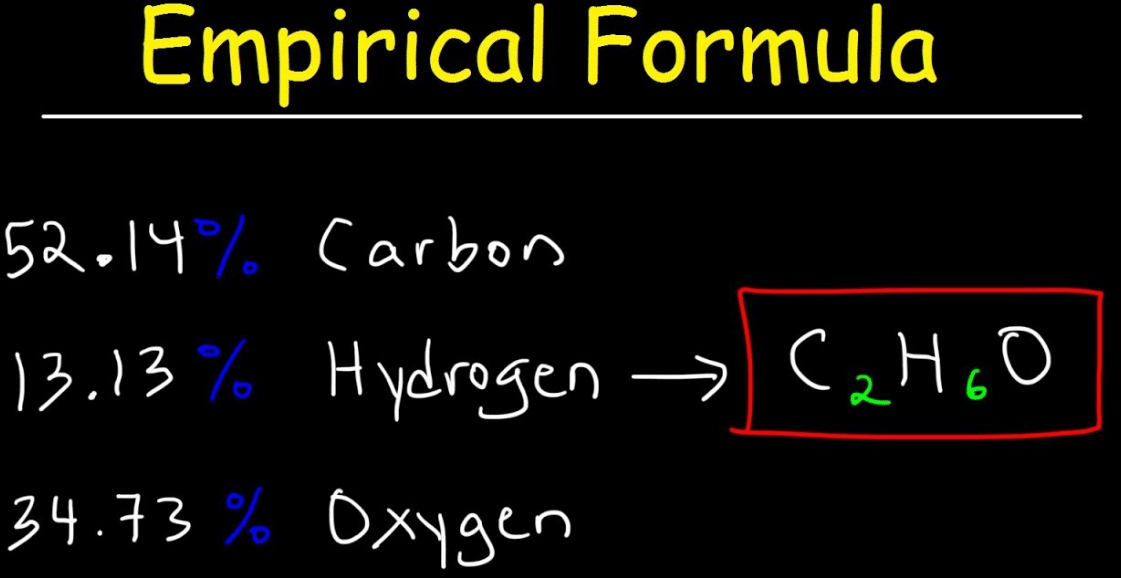
Empirical Formula Definition Get Education
https://geteducationskills.com/wp-content/uploads/2020/03/Empirical-Formula-Definition-.png
Oct 12 2020 nbsp 0183 32 When a new chemical compound such as a potential new pharmaceutical is synthesized in the laboratory or isolated from a natural source chemists determine its elemental composition its empirical formula and its structure to understand its properties There are two types of formulas empirical and molecular Empirical Formula Lowest whole number ratio of the e lements in a compound Molecular Formula Actual whole number ratio of the elements in a compound
Molecular formulas are derived by comparing the compound s molecular or molar mass to its empirical formula mass As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula Empirical formulae and molecular formulae A compound can be represented by two types of chemical formulae Empirical formula of a compound gives the simplest whole number ratio of atoms of each element present in the compound Molecular formula of a compound gives the actual number of atoms of each element present in one molecule of the compound
More picture related to What Is Empirical And Molecular Formula

Difference Between Empirical And Molecular Formula Class 11 Chemistry
https://i.ytimg.com/vi/0yp20L7_XzU/maxresdefault.jpg
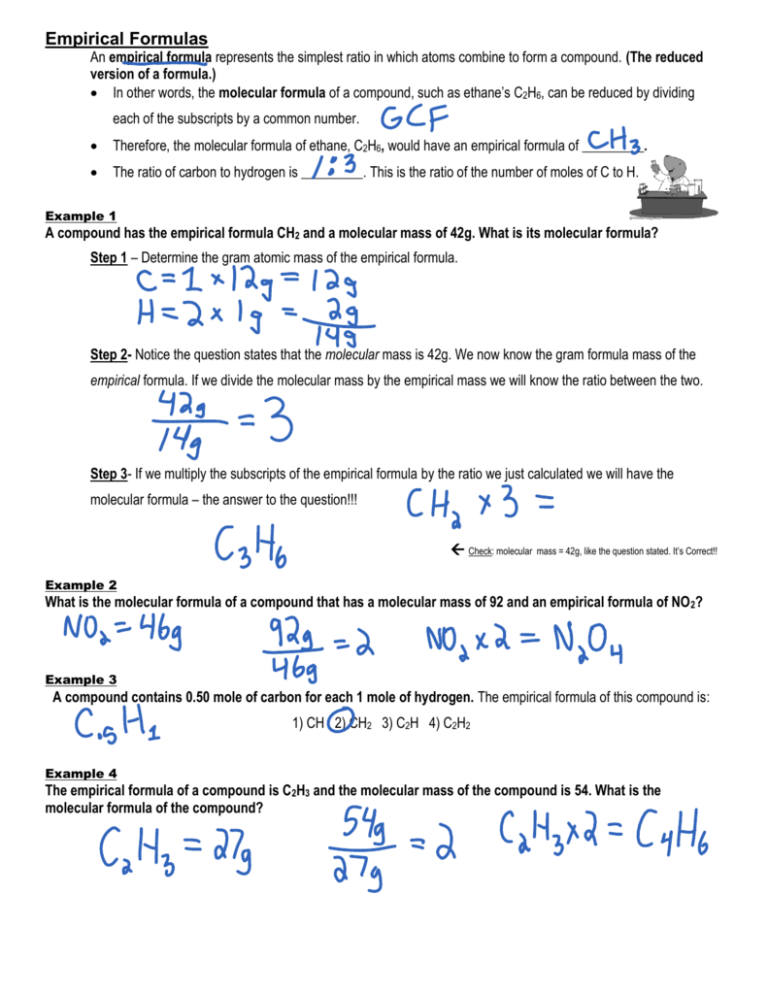
Empirical Formulas
https://s3.studylib.net/store/data/008797804_1-ddfb6a87ec9d9750facfc6078e5d8d18-768x994.png
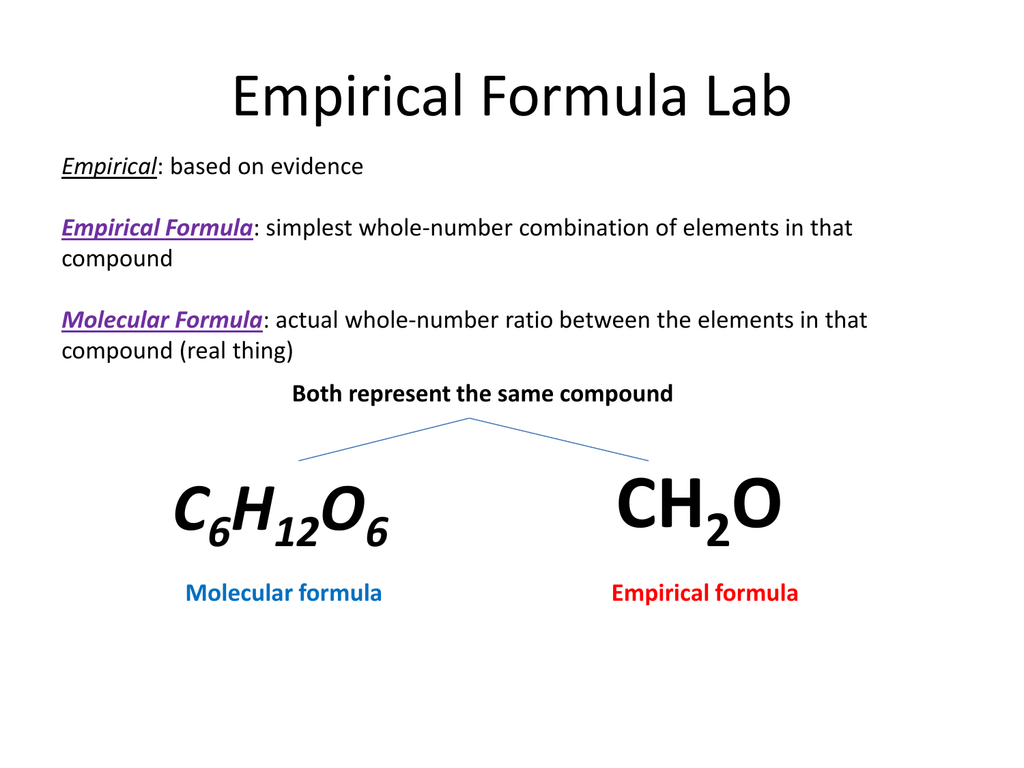
Empirical Formula Lab
https://s2.studylib.net/store/data/005789747_1-51986e07be6aa405206a09f12567cfe2.png
In this tutorial you will learn what an empirical formula and molecular formula are and the differences of molecular formula vs empirical formula You will also learn how to convert between the molecular and empirical formula Nov 25 2021 nbsp 0183 32 An empirical formula represents the simplest ratio of elements in a compound while a molecular formula provides the actual number of atoms of each element in a molecule
Jan 18 2025 nbsp 0183 32 Calculating Empirical Formula The empirical formula is the simplest whole number ratio of the atoms of each element present in one molecule or formula unit of the compound E g the empirical formula of ethanoic acid is CH 2 O Organic molecules often have different empirical and molecular formulae The formula of an ionic compound is always Sep 29 2024 nbsp 0183 32 The empirical formula of an organic molecule is often different to its molecular chemical formula For example ethanoic acid has the chemical formula CH 3 COOH or C 2 H 4 O 2 but its empirical formula is CH 2 O The molecular chemical formula of an ionic compound is always its empirical formula For example sodium chloride has the chemical

Empirical Vs Molecular Formula
https://sciencenotes.org/wp-content/uploads/2020/08/O5-1024x683.png

Question Video Determining An Empirical Formula Given The Ratio Of
https://media.nagwa.com/524156373186/en/thumbnail_l.jpeg
What Is Empirical And Molecular Formula - Empirical formulae and molecular formulae A compound can be represented by two types of chemical formulae Empirical formula of a compound gives the simplest whole number ratio of atoms of each element present in the compound Molecular formula of a compound gives the actual number of atoms of each element present in one molecule of the compound