What Factor Affects The Rate Of Chemical Reaction When The Kindling Is Used To Start A Fire The rate of chemical reactions is affected by factors such as temperature concentration and particle size To put it simply temperature and concentration are directly proportional and particle size is indirectly proportional to the rate of chemical reactions
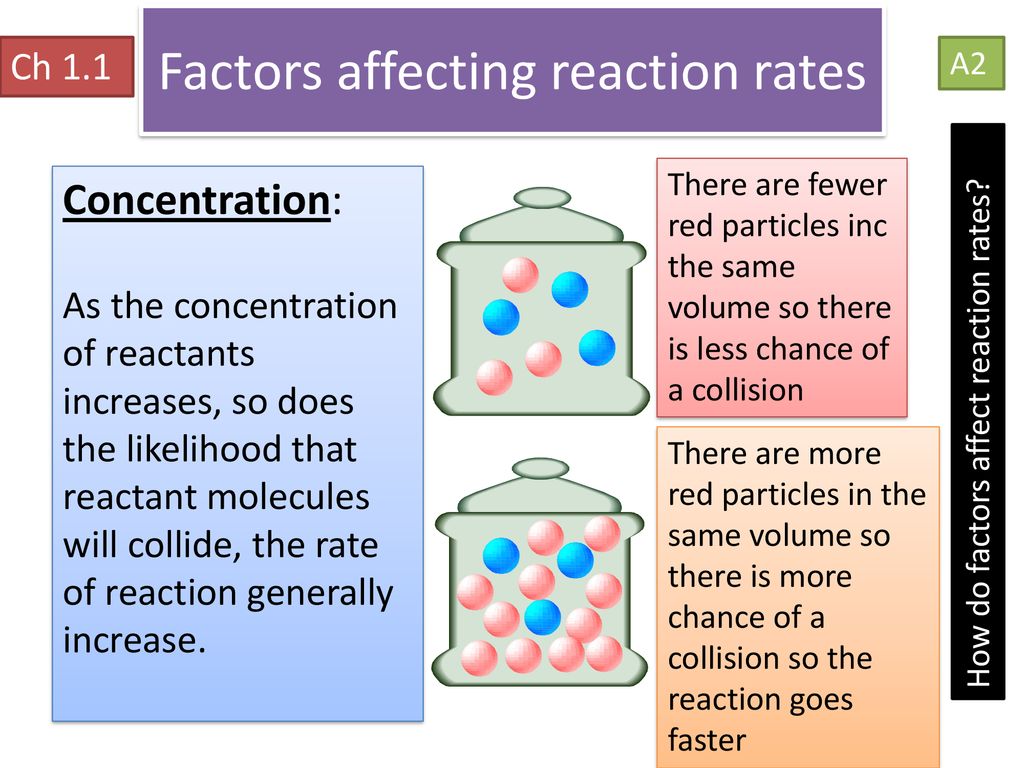
Rate of a reaction is directly proportional to the concentration of reactants i e rate r c n where or r kc n where Explanation The number of collisions and hence the activated collisions between the reactant molecules increase with increase in concentration May 25 2021 nbsp 0183 32 Chemists have identified many factors that affect the rate of a reaction The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation
What Factor Affects The Rate Of Chemical Reaction When The Kindling Is Used To Start A Fire

What Factor Affects The Rate Of Chemical Reaction When The Kindling Is Used To Start A Fire
https://i.ytimg.com/vi/-4HXaUBbv04/maxresdefault.jpg
Factors Affecting The Rate Of The Reaction Chemical Kinetics YouTube
https://i.ytimg.com/vi/JpoOfrPKgmM/maxresdefault.jpg

Factors Affecting The Rate Of Chemical Reaction Every Factor
https://i.ytimg.com/vi/QQeDNVijlpA/maxresdefault.jpg
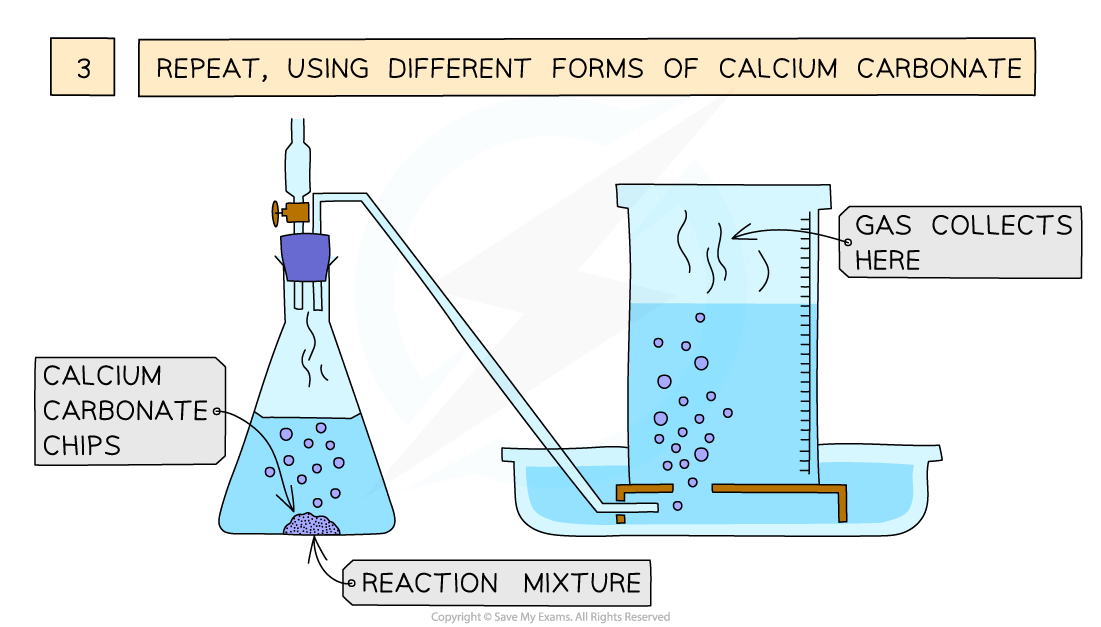
Mar 27 2024 nbsp 0183 32 You need to be able to describe how decreasing one of the factors affects the rate of a chemical reaction For example decreasing temperature means that reactant particles have less kinetic energy resulting in less frequent successful collisions and a slower rate of reaction According to the collision theory the rate of reaction increases with the increase in the concentration of the reactants As per the law of mass action the chemical reaction rate is directly proportional to the concentration of reactants
The rate of a chemical reaction is affected by several parameters Reactions involving two phases proceed more rapidly when there is greater surface area contact If temperature or reactant concentration is increased the rate of a given reaction generally increases as well There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the
More picture related to What Factor Affects The Rate Of Chemical Reaction When The Kindling Is Used To Start A Fire

The Virtual Science Laboratories VSLs
https://csmis.reb.rw/vsl/assets/images/chemical.jpeg
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 3 2 4 Practical Effect Of
https://oss.linstitute.net/wechatimg/2022/11/3.2.1-Effect-of-Surface-Area-on-a-Reaction-Rate-3.png
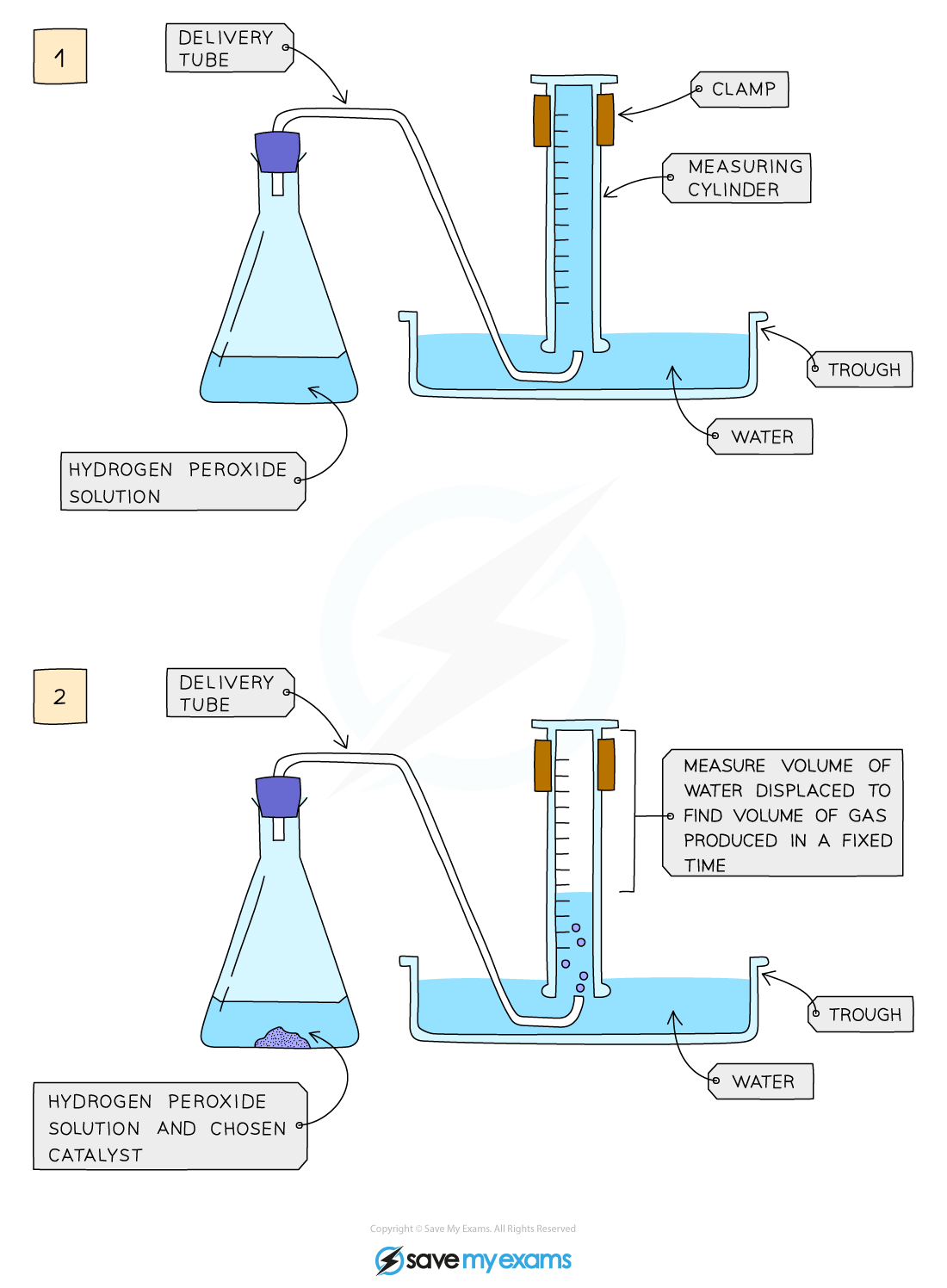
Edexcel IGCSE Chemistry 3 2 6 Practical Effect Of Catalysts On
https://oss.linstitute.net/wechatimg/2022/07/Investigating-effect-of-catalyst-on-rate-reaction-3.png
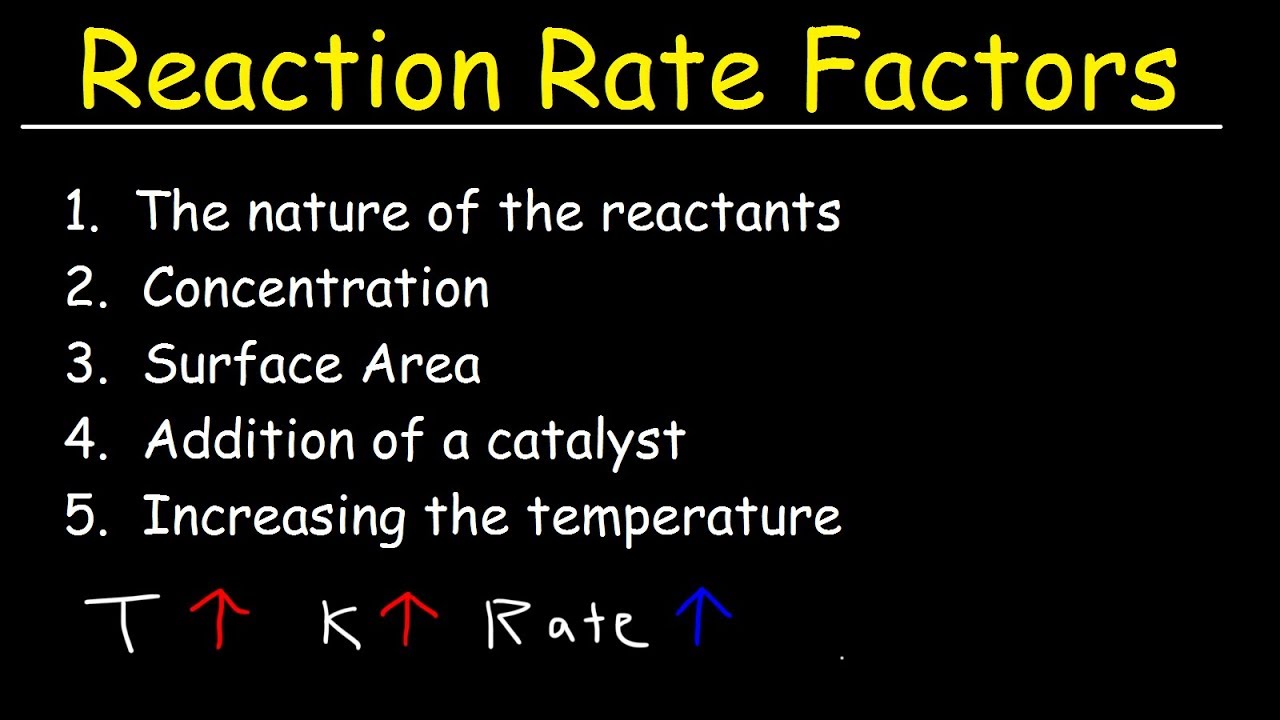
There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction Reactions occur when two reactant molecules effectively collide each having minimum energy and correct orientation Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are
What factors affect the rate of a chemical reaction The four main factors that affect the rate of chemical reactions are Concentration of the reactants Temperature of the reactants Surface area of the reactants Addition of a Catalyst 1 Effect of Concentration on reaction rate Nov 20 2024 nbsp 0183 32 Increasing the concentration of a solution or gas pressure increases the rate of reaction Increasing the temperature increases the rate of reaction Increasing the surface area increases the rate of reaction Surface area increases as particle size decreases
Chapter 1 Rate Of Reaction Ppt Download
https://slideplayer.com/slide/15098838/91/images/6/Factors+affecting+reaction+rates.jpg

Factors Affecting The Rate Of Chemical Reaction Online Presentation
https://cf.ppt-online.org/files/slide/i/ISHfNCBKgTAkQn452mvbL1PshMxJzjtcya7EdZ/slide-0.jpg
What Factor Affects The Rate Of Chemical Reaction When The Kindling Is Used To Start A Fire - The rate of a chemical reaction is affected by several parameters Reactions involving two phases proceed more rapidly when there is greater surface area contact If temperature or reactant concentration is increased the rate of a given reaction generally increases as well