What Does Rate Of Reaction Depend On There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst
Oct 29 2020 nbsp 0183 32 Here is a list of factors that affect reaction rate an explanation of why they work and a look at the limitations of increasing rate Reactants in the same state of matter react more readily than those in different phases Mixing helps improve reaction rate Aug 14 2024 nbsp 0183 32 The Rate of Reaction depends on the Activation Energy in the manner that if the Activation Energy is high then the Rate of Reaction will be low and vice versa Hence Activation Energy and Rate of Reaction are inversely related to each other
What Does Rate Of Reaction Depend On

What Does Rate Of Reaction Depend On
https://i.ytimg.com/vi/QjcMgk45sOQ/maxresdefault.jpg

GCSE Chemistry How To Calculate The Rate Of Reaction Measuring Rate
https://i.ytimg.com/vi/GCR5xeduq2o/maxresdefault.jpg

Determine The Rate Constant k For A Reaction YouTube
https://i.ytimg.com/vi/8KuSMRycbYg/maxresdefault.jpg
The rate of chemical reactions depends on the temperature concentration of the reactants size of the particles reacting and whether there are any catalysts present When the temperature is increased the average kinetic energy of the particles present is increased The reaction rate or rate of reaction is the speed at which a chemical reaction takes place defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time 1 Reaction rates can vary dramatically
Sep 16 2022 nbsp 0183 32 The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation Nov 20 2024 nbsp 0183 32 Increasing the temperature increases the rate of reaction Explanation Compared to a reaction at a low temperature the line graph for the same reaction at a higher temperature Has a steeper gradient at the start Becomes horizontal sooner Forms the same amount of product This shows that increasing the temperature increases the rate of reaction
More picture related to What Does Rate Of Reaction Depend On
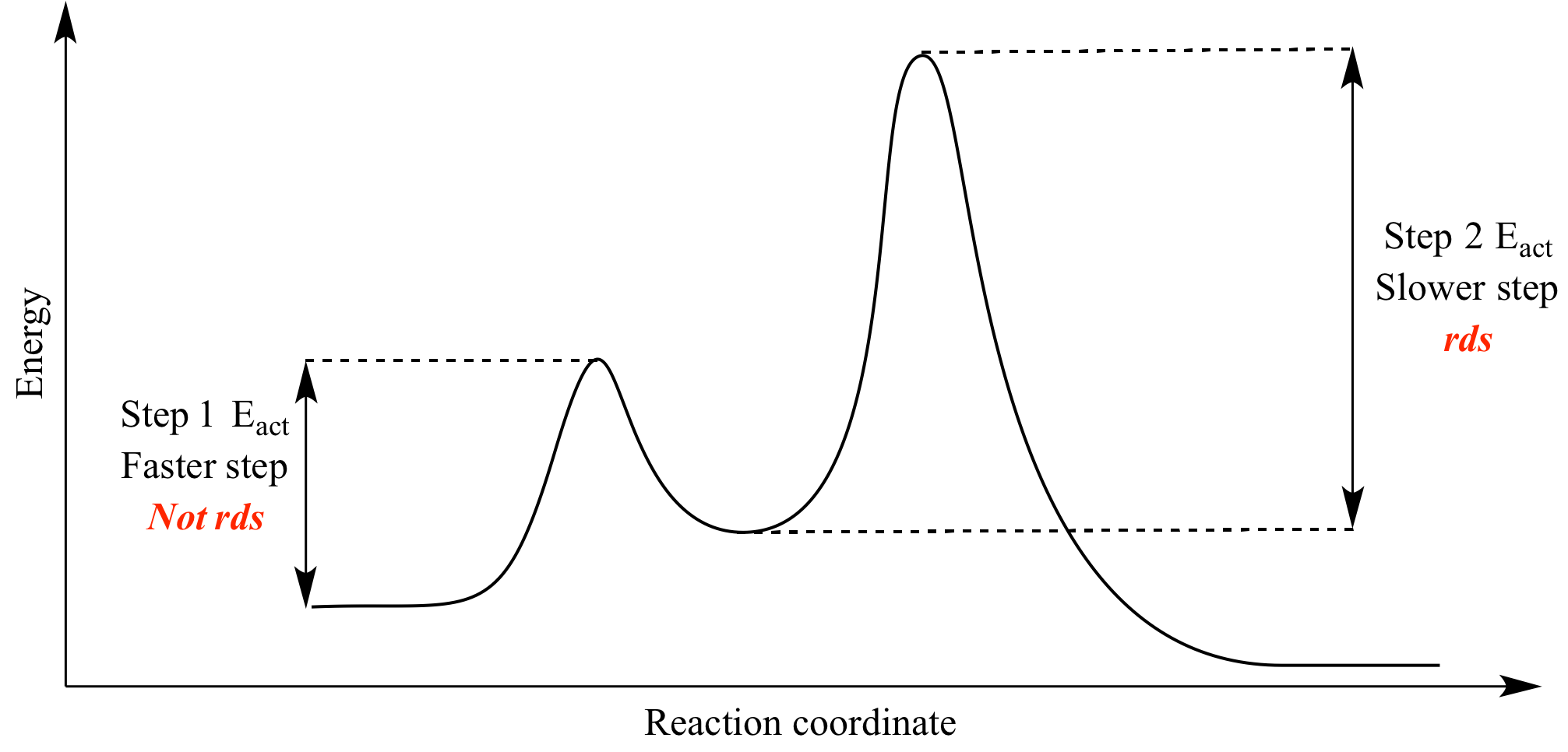
Illustrated Glossary Of Organic Chemistry Rate Determing Step
http://www.chem.ucla.edu/~harding/IGOC/R/rate_determining_step01.png
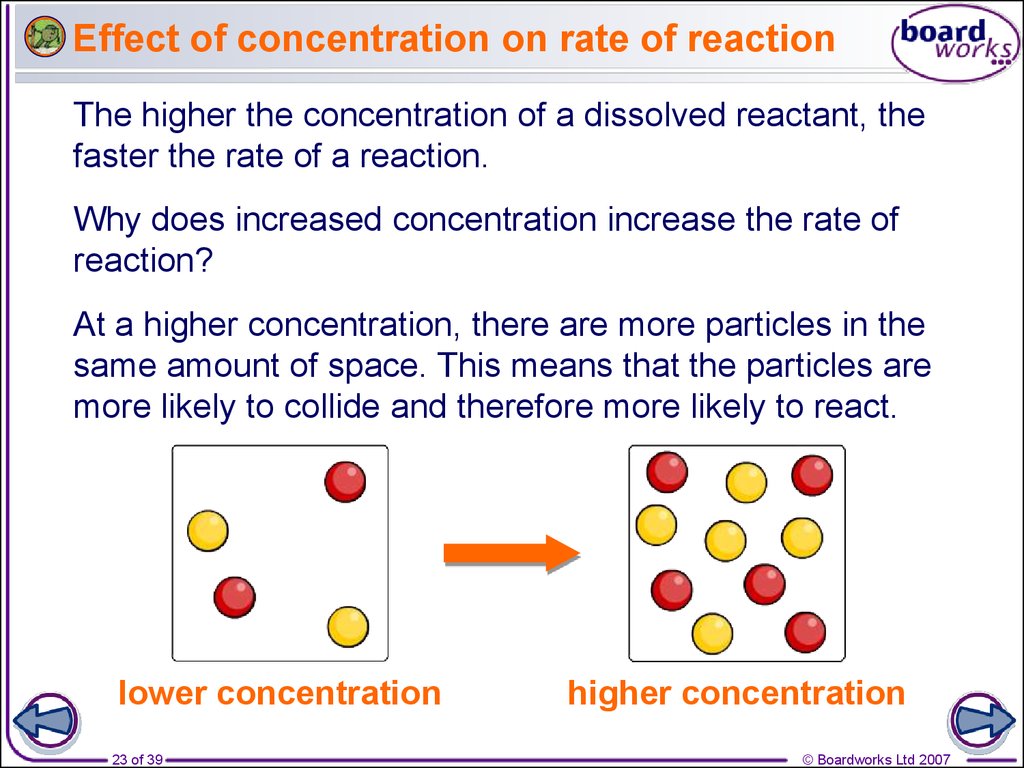
Rates Of Reaction
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-22.jpg
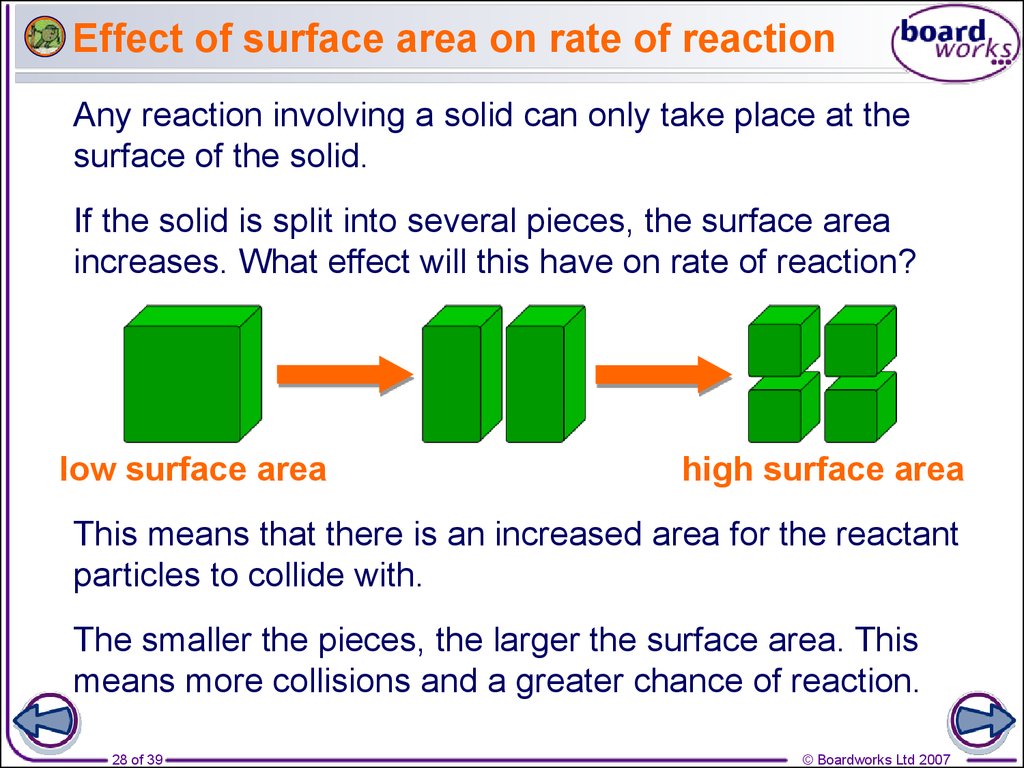
Rates Of Reaction
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-27.jpg
The rate of a chemical reaction is proportional to concentration of reactants present As reactants are used up during the process the rate will decrease and the reaction slows down The rate of a chemical reaction or the reaction rate can be defined by the time needed for a change in concentration to occur But there is a problem in that this allows for the definition to be made based on concentration changes for either the reactants or the products
There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction May 12 2018 nbsp 0183 32 The rate of reaction in general varies directly with changes in the concentration of the reactants When the concentration of all the reactants increases more molecules or ions interact to form new compounds and the rate of reaction increases

Following Directions Worksheets Activities For Beginners Good
https://worksheets.clipart-library.com/images2/following-directions-worksheet-funny/following-directions-worksheet-funny-35.png
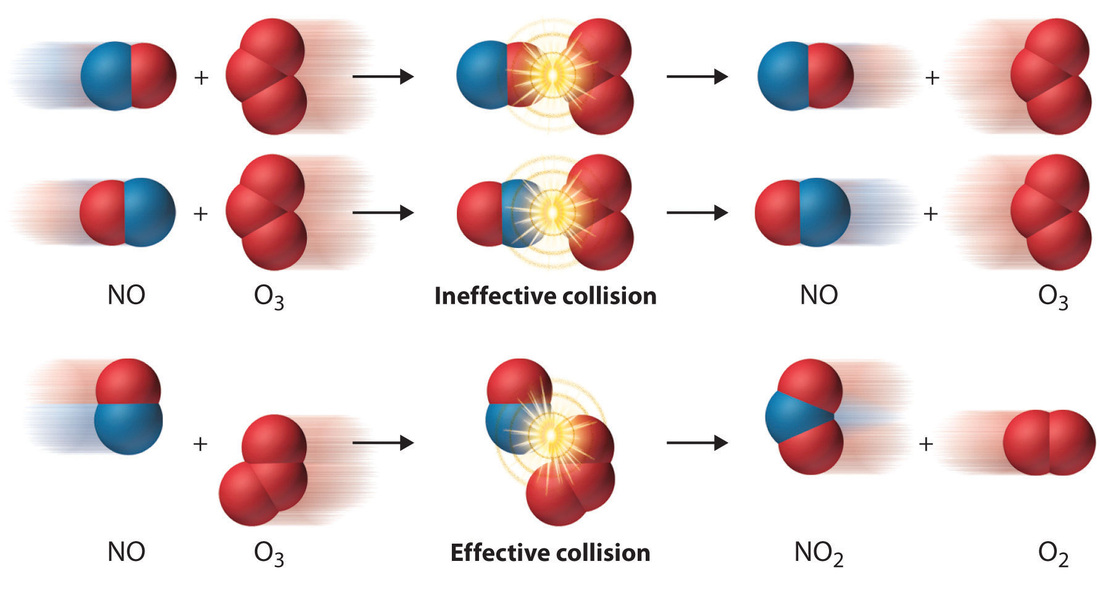
C12 Rates Of Reaction Mr Tremblay s Class Site
http://mrtremblaycambridge.weebly.com/uploads/9/7/8/8/9788395/5001988_orig.jpg
What Does Rate Of Reaction Depend On - A catalyst generally increases the speed of a reaction without itself being consumed in the reaction In case of reverse reactions a catalyst helps to attain the equilibrium quickly without disturbing the state of equilibrium