What Decreases The Rate Of Chemical Reaction As per the law of mass action the chemical reaction rate is directly proportional to the concentration of reactants This implies that the chemical reaction rate increases with the
May 30 2014 nbsp 0183 32 There are a few ways to decrease the rate of reaction which I shall mention Perhaps the most obvious answer is to decrease the temperature of the reaction this slows May 12 2018 nbsp 0183 32 Increasing the concentration of reactants generally increases the rate of reaction because more of the reacting molecules or ions are present to form the reaction products This
What Decreases The Rate Of Chemical Reaction

What Decreases The Rate Of Chemical Reaction
https://slideplayer.com/slide/13266359/79/images/8/Moisture+The+rate+of+chemical+reactions+and+mechanical+weathering+increase+when+water+is+present..jpg
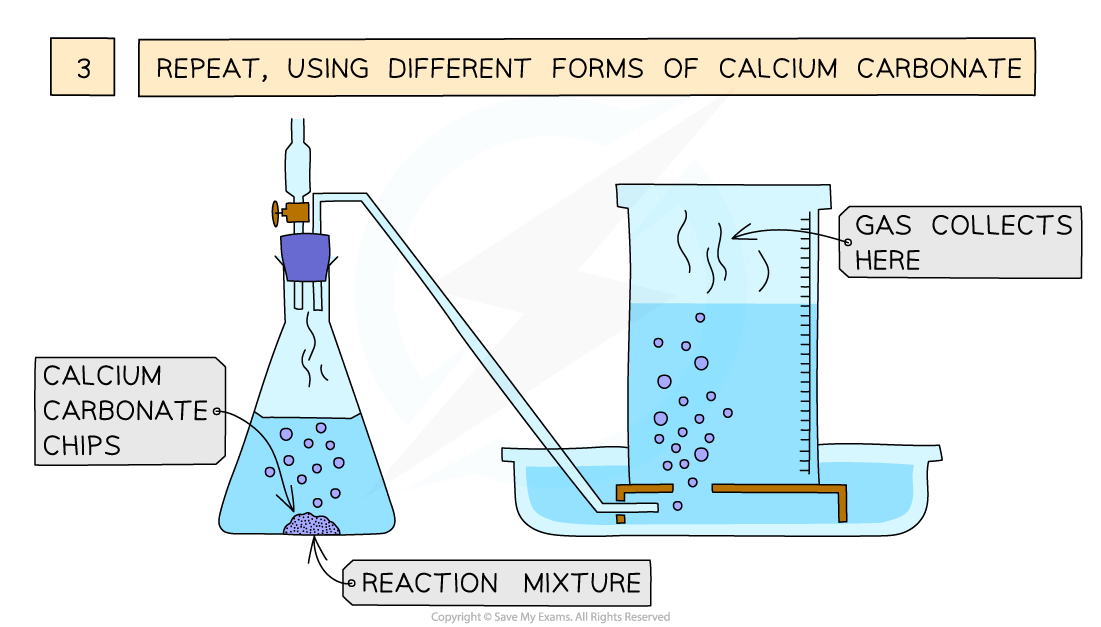
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 3 2 4 Practical Effect Of
https://oss.linstitute.net/wechatimg/2022/11/3.2.1-Effect-of-Surface-Area-on-a-Reaction-Rate-3.png

Reaction Rate Equation Download Table
https://www.researchgate.net/profile/Hossein-Mohammadiarani/publication/295744274/figure/tbl1/AS:670040223391759@1536761446638/Reaction-rate-equation.png
Sep 24 2024 nbsp 0183 32 Increasing the concentration of a solution or gas pressure increases the rate of reaction Increasing the surface area increases the rate of reaction Surface area increases as There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion
Sep 16 2022 nbsp 0183 32 The rate of reaction is proportional to the number of collisions over time increasing the concentration of either reactant increases the number of collisions and therefore increases The rate of a chemical reaction is proportional to concentration of reactants present As reactants are used up during the process the rate will decrease and the reaction slows down
More picture related to What Decreases The Rate Of Chemical Reaction
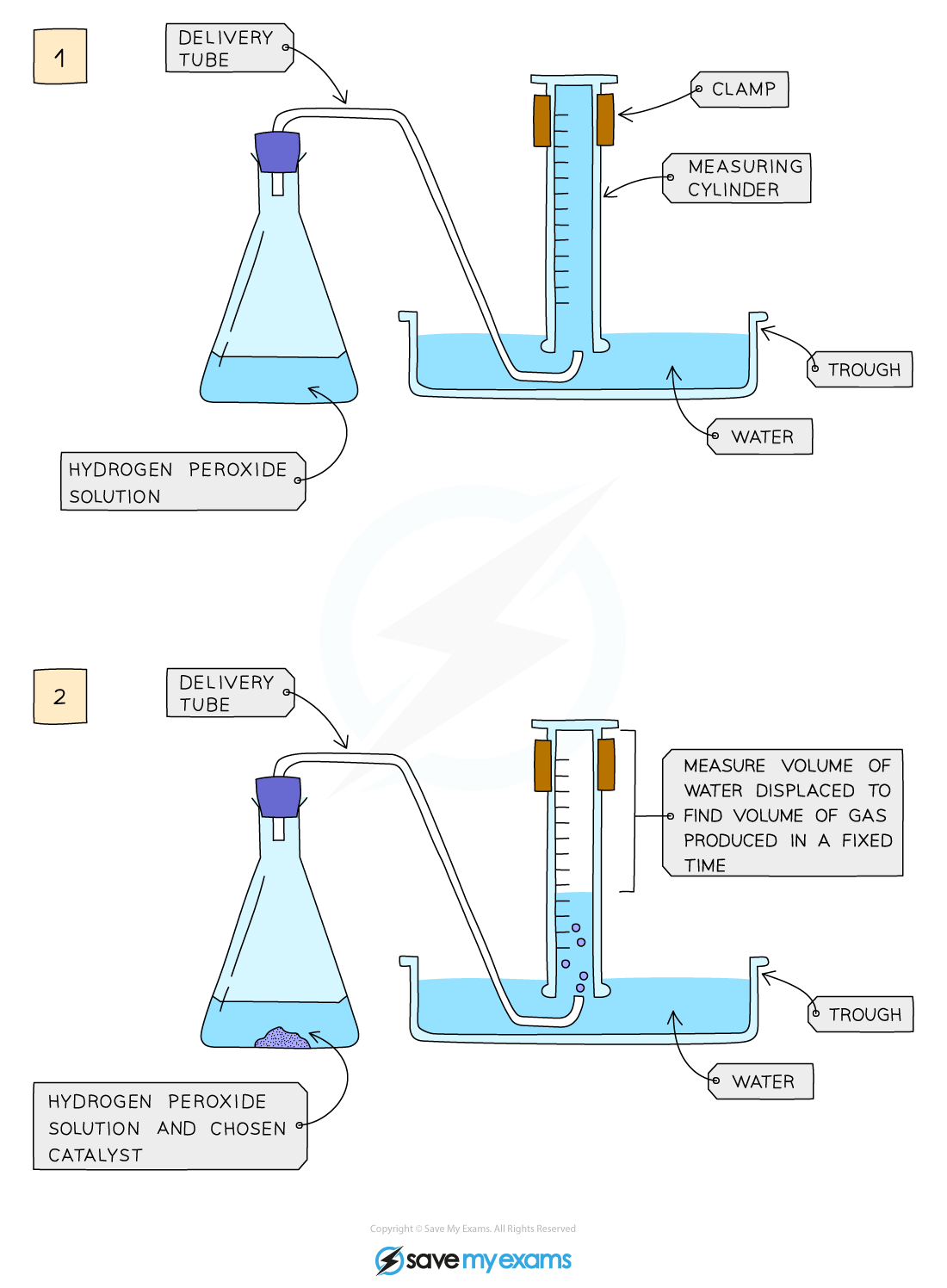
Edexcel IGCSE Chemistry 3 2 6 Practical Effect Of Catalysts On
https://oss.linstitute.net/wechatimg/2022/07/Investigating-effect-of-catalyst-on-rate-reaction-3.png
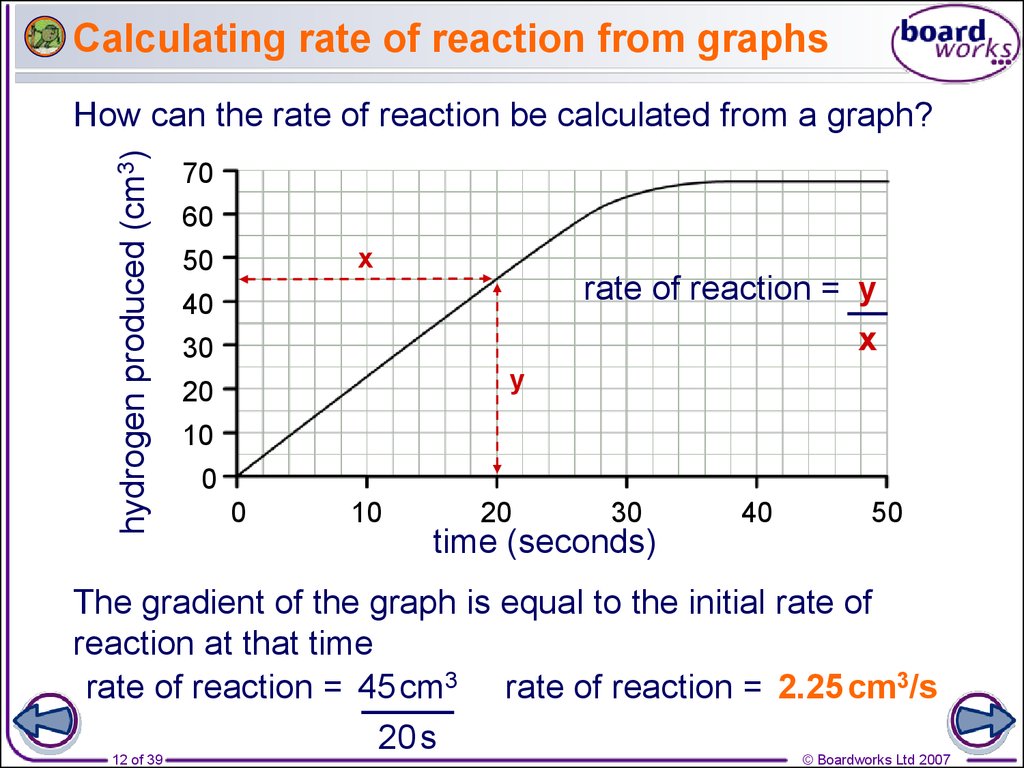
Rates Of Reaction
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-11.jpg
+reaction%3B.jpg)
ENZYMES AHL Topic 7 6 IB Biology Miss Werba Ppt Download
https://slideplayer.com/slide/15796635/88/images/27/Sample+questions+they+increase+rate+of+(chemical)+reaction%3B.jpg
Chemical reaction rates increase or decrease according to factors including temperature pressure and light A catalyst changes the rate of reaction but is unchanged at the end of the Feb 16 2020 nbsp 0183 32 Chemical reactions take place when particles collide with enough energy and in the correct orientation Without collisions no reaction is possible The rate of reaction is
Jul 12 2023 nbsp 0183 32 In this section we will show you how to quantitatively determine the reaction rate Typically reaction rates decrease with time because reactant concentrations decrease as There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at
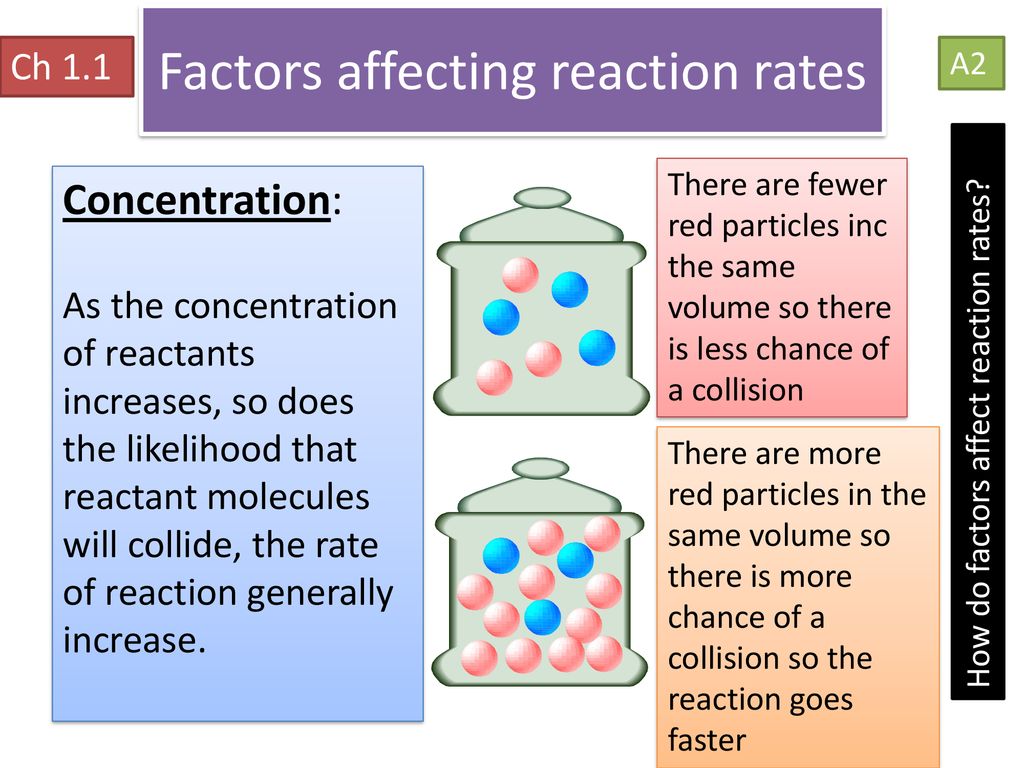
Chapter 1 Rate Of Reaction Ppt Download
https://slideplayer.com/slide/15098838/91/images/6/Factors+affecting+reaction+rates.jpg

Question Video Determining The Factor That Does Not Affect The Rate Of
https://media.nagwa.com/480109852128/en/thumbnail_l.jpeg
What Decreases The Rate Of Chemical Reaction - The rate of a chemical reaction is proportional to concentration of reactants present As reactants are used up during the process the rate will decrease and the reaction slows down