What Are The Specific Heat Capacity In thermodynamics the specific heat capacity symbol c of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature It is also referred to as massic heat capacity or as the specific heat
Unlike the total heat capacity the specific heat capacity is independent of mass or volume It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree Celsius The units of specific heat capacity are J kg 176 C or equivalently J kg K Define heat capacity and specific heat capacity and differentiate between the two terms Deduce which substance will have greatest temperature changed based on specific heat capacities Calculate unknown variables based on known
What Are The Specific Heat Capacity

What Are The Specific Heat Capacity
https://adilhakeem2007.files.wordpress.com/2021/09/whatsapp-image-2021-09-09-at-8.51.15-pm.jpeg
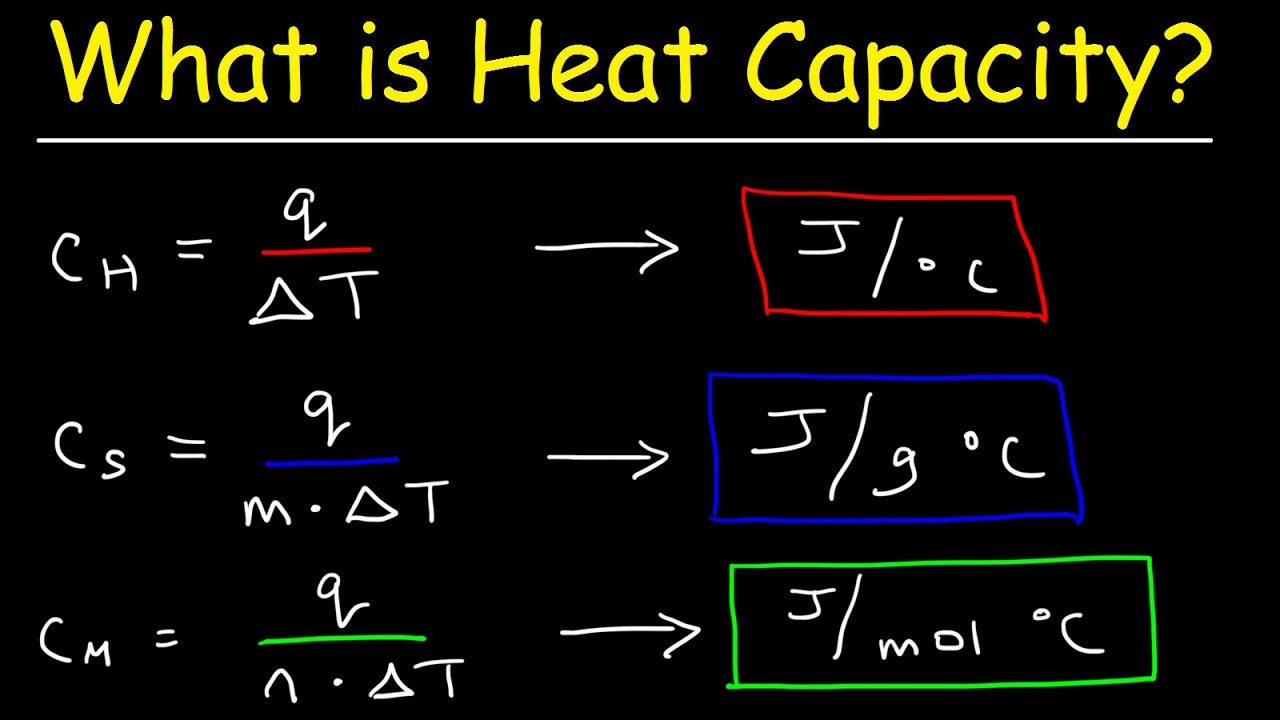
What Is The Difference Between Specific Heat Capacity Heat Capacity
https://i.ytimg.com/vi/IoHXMaiwT80/maxresdefault.jpg
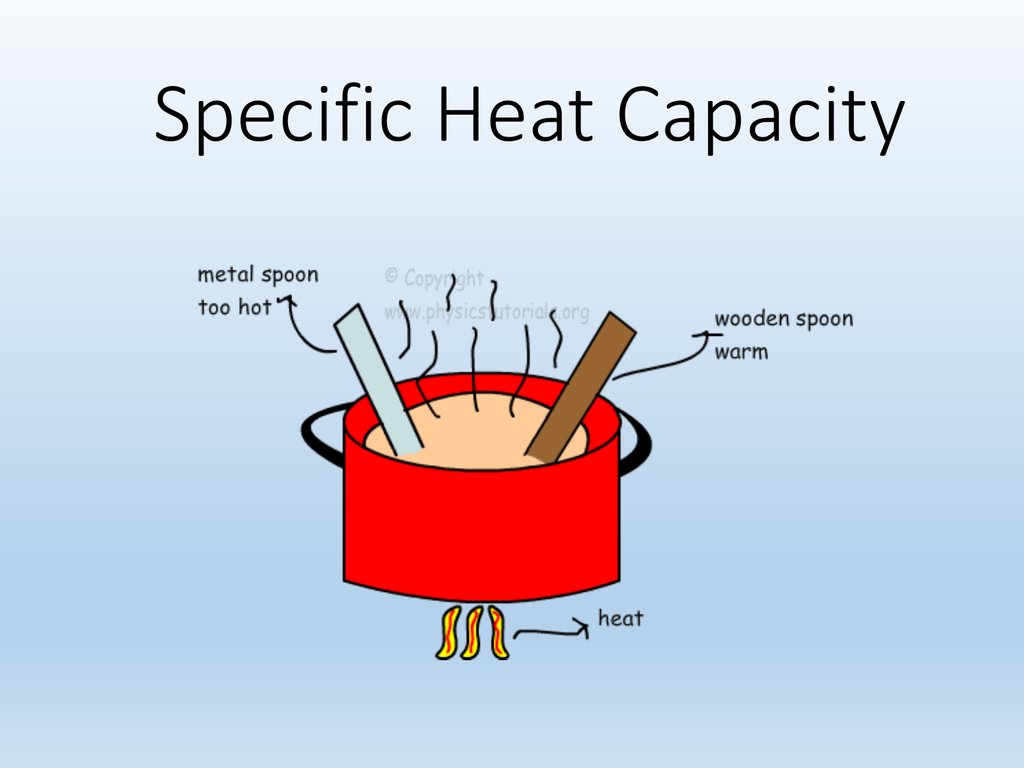
1 3 Specific Heat Capacity
https://s2.studylib.net/store/data/018037878_1-5dc8c711621f05e80a2a3975e8530b10.png
Aug 14 2024 nbsp 0183 32 Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass The specific heat capacity of a material is a physical property as well as an extensive property since its value is proportional to the size of the system being examined Aug 29 2022 nbsp 0183 32 Heat capacity is the amount of heat required to raise the temperature of an object by 1 text o text C The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1 text o text C
Specific heat the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree The units of specific heat are usually calories or joules per gram per Celsius degree The specific heat of water is 1 calorie Jul 30 2024 nbsp 0183 32 The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 176 C The formula is Cv Q T 215 m
More picture related to What Are The Specific Heat Capacity

Change In ENTHALPY Using Specific Heat At Constant Pressure In 3
https://i.ytimg.com/vi/OUbEHKNYFwg/maxresdefault.jpg
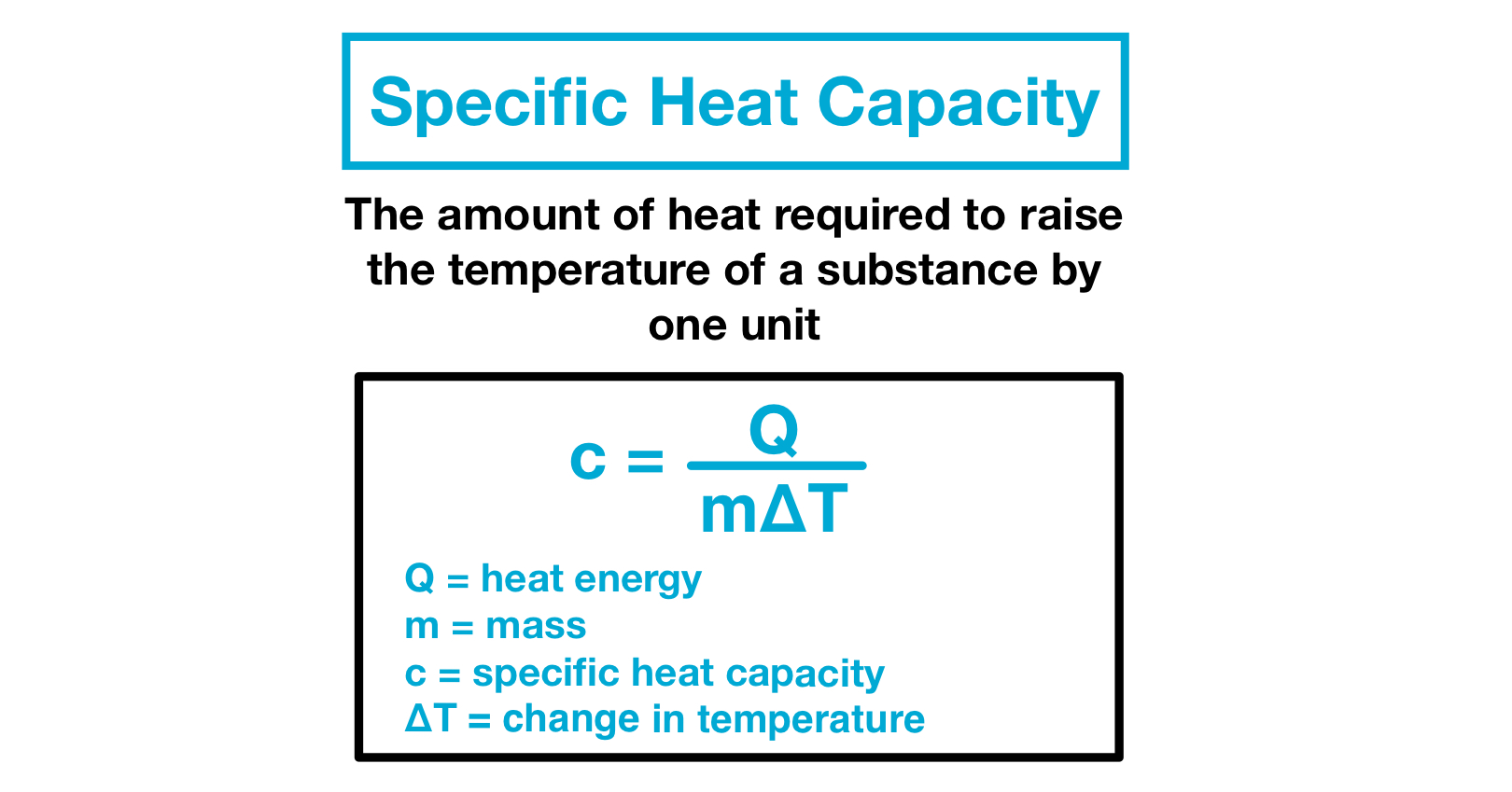
Specific Heat Capacity Definition AbramsrEwing
https://d20khd7ddkh5ls.cloudfront.net/heat_capacity.jpeg

PPT Specific Heat Capacity PowerPoint Presentation Free Download
https://image1.slideserve.com/3439007/measuring-specific-heat-capacity1-l.jpg
The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius 176 C Specific heat is closely related to the concept of heat capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1 00 176 C 176 C In equation form heat capacity C is C m c C m c where m is mass and c is specific heat
May 13 2023 nbsp 0183 32 The specific heat capacity c of a substance commonly called its specific heat is the quantity of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius or 1 kelvin The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1 176 C

Specific Heat Capacity Of Water Experiment GCSE Physics Required
https://i.ytimg.com/vi/7bucHPbrxkg/maxresdefault.jpg
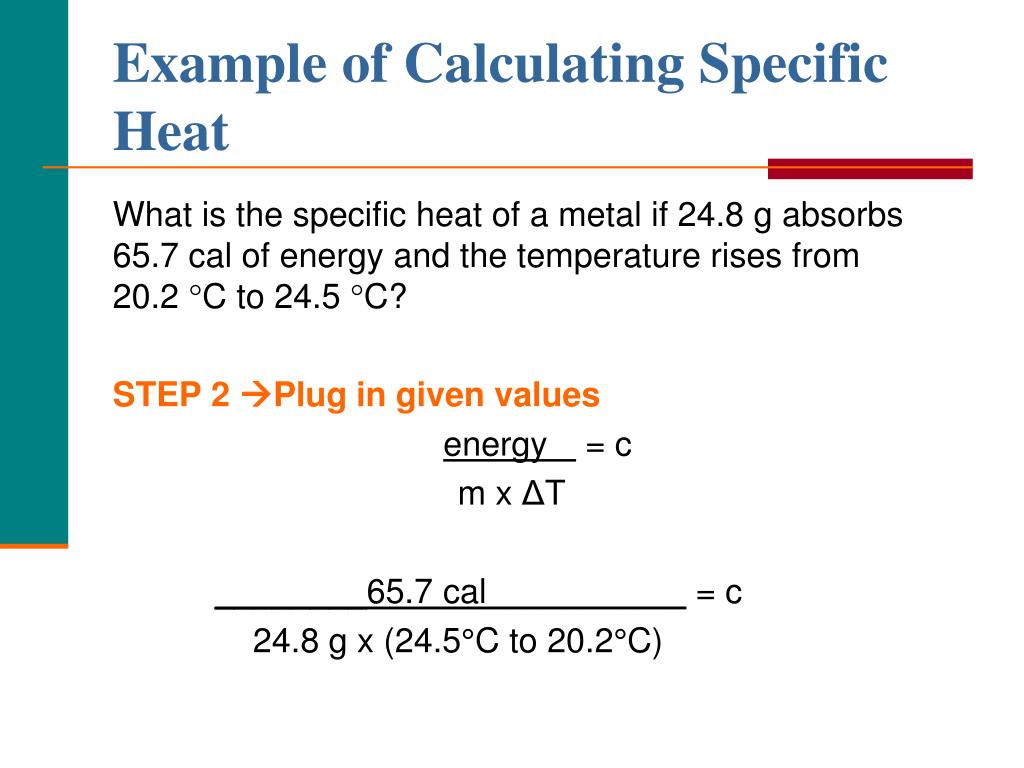
How To Calculate Specific Heat Capacity Calculator Haiper
https://image2.slideserve.com/3721637/example-of-calculating-specific-heat1-l.jpg
What Are The Specific Heat Capacity - Aug 29 2022 nbsp 0183 32 Heat capacity is the amount of heat required to raise the temperature of an object by 1 text o text C The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1 text o text C