Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers But they don t have to be Here is an example of a mass mass stoichiometric problem based on the relationships within one chemical substance Solution 1 Determine moles of calcium 66 0 g 40 078 g mol 1 6468 mol 2 Determine moles of oxygen in the sample based on a 3 8 ratio between Ca and O 3 8
We can use that ability to answer stoichiometry questions in terms of the masses of a particular substance in addition to moles We do this using the following sequence Collectively these conversions are called mole mass calculations As an example consider the balanced chemical equation The mol Fe 2 O 3 units cancel leaving mol SO 3 unit Stoichiometry Worksheet 2 mole mass mass mole problems 1 N 2 2O 2 N 2 O 4 a If 15 0g of N 2 O 4 What is the mass of potassium nitrate that is produced when 2 04 moles of potassium phosphate react 2 Balance and answer the following questions a How many grams of NaCl are produced when 20 00mol of NaClO 3
Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers

Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers
https://i.ytimg.com/vi/opb1Z75rcWI/maxresdefault.jpg
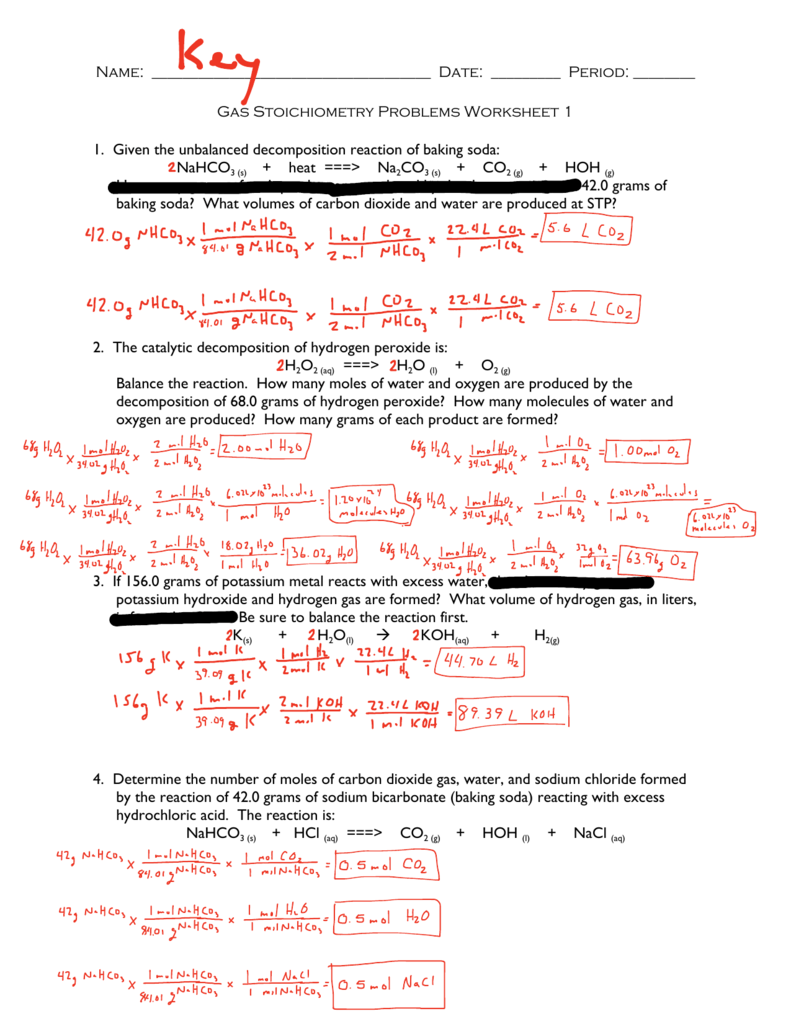
Gas Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png
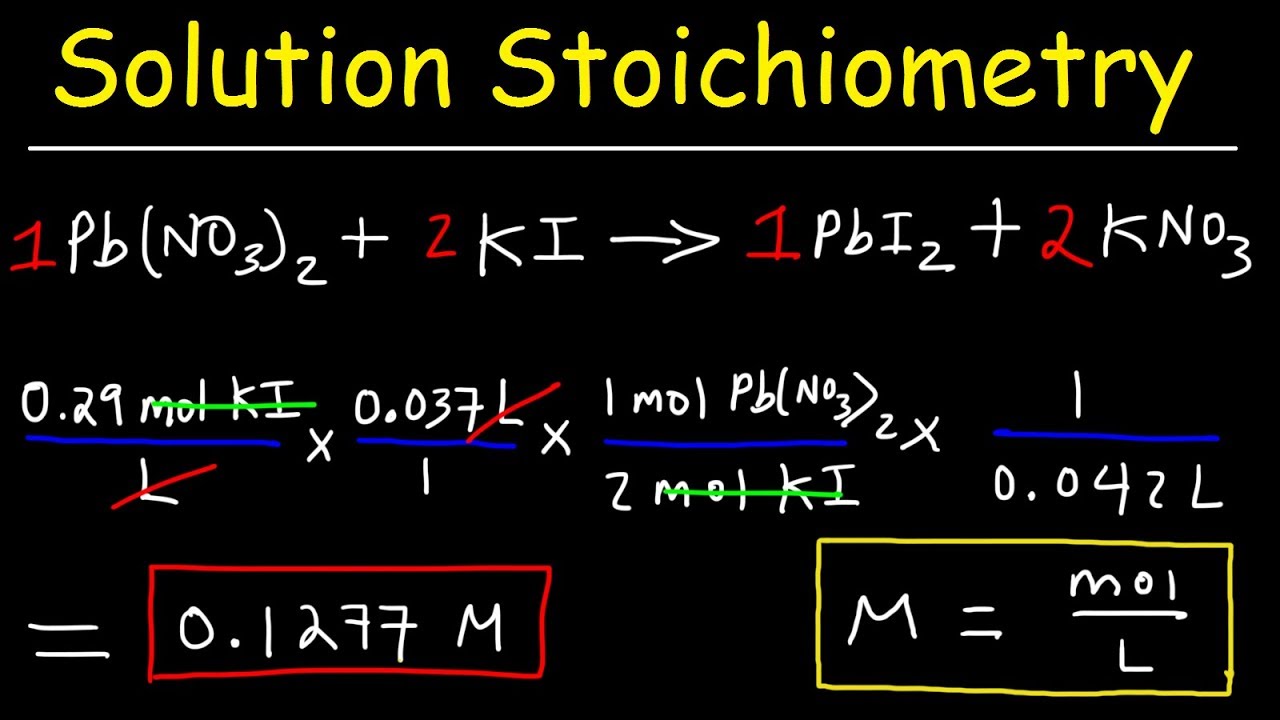
Solution Stoichiometry Finding Molarity Mass Volume YouTube
https://i.ytimg.com/vi/Ab3wfKjaWWQ/maxresdefault.jpg
Flowchart of steps in stoichiometric calculations Step 1 grams of A is converted to moles by multiplying by the inverse of the molar mass Step 2 moles of A is converted to moles of B by multiplying by the molar ratio Step 3 moles of B is converted to grams of B by the molar mass STOICHIOMETRY WORKSHEET 1 MASS MASS 1 Determine the mass of lithium hydroxide produced when 0 38 grams of lithium nitride reacts with water according to the following unbalanced chemical equation Li 3N s H 2O l NH 3 g LiOH aq 2 What mass of sodium chloride is produced when chlorine gas reacts with 0 29 grams of sodium iodide
Mole mass calculation where we start with a given number of moles of a substance and calculate the mass of another substance involved in the chemical equation or vice versa For example suppose we have the balanced chemical equation 2Al 3Cl 2 2AlCl 3 Suppose we know we have 123 2 g of Cl 2 Learn how to use a balanced chemical equation to calculate the amounts of reactants and products involved in a chemical reaction This chapter explains the concepts and methods of reaction stoichiometry including mole mole mass mass and limiting reactant calculations Explore examples and exercises from the OpenStax Chemistry 2e textbook
More picture related to Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers
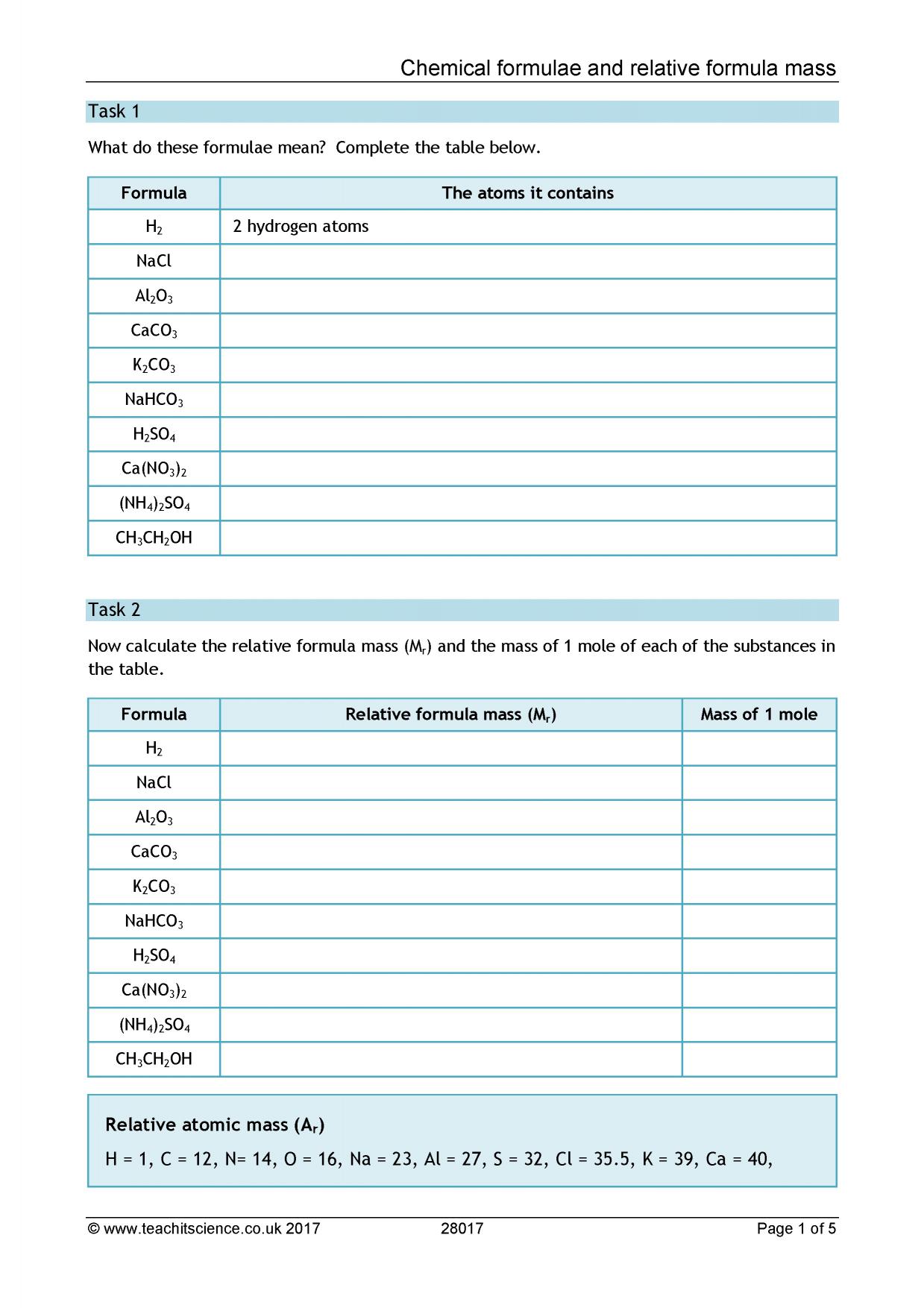
Chemical Formulae And Relative Mass Worksheet KS4 Chemistry Teachit
https://www.teachit.co.uk/sites/default/files/products/m_thumbnails/28017/28017_generated.jpeg
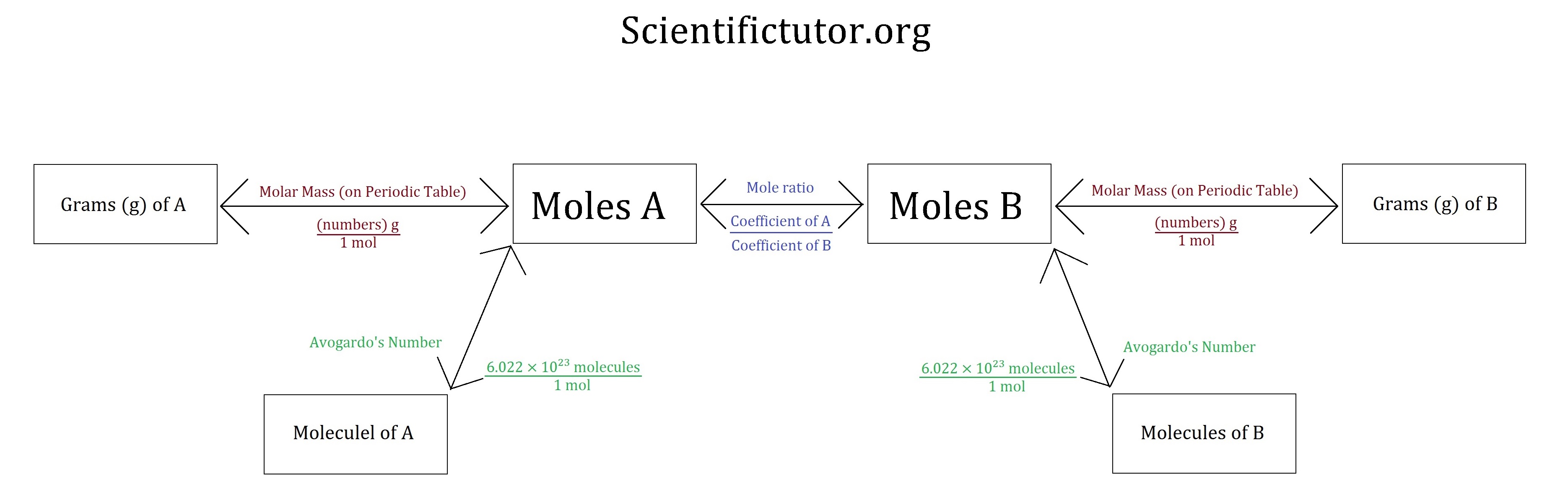
Using Stoichiometry In Conversions Scientific Tutor
http://scientifictutor.org/wp-content/uploads/2016/10/Conversion-Diagram-Stoichiometry-2.jpg

164 Stoichiometric Practice Stoichiometric Calculations
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/569ef88a33f7fe836e34bb237fa1832b/thumb_1200_1553.png
Apply mass to mass stoichiometry Table salt or sodium chloride can be decomposed into sodium metal and chlorine gas by running electricity through molten NaCl A Downs cell produces sodium and chlorine from molten sodium chloride If 42 8 grams of Cl A 2 are produced by the above reaction what mass of NaCl reacted For example to find the molar mass of eq H 2O eq find the mass of two hydrogen atoms and one oxygen atom and add them together The mass of each mole of hydrogen atoms is 1 008 g so
Chapter 3 Stoichiometry Michigan State UniversityLearn the basic concepts and calculations of stoichiometry the quantitative study of chemical reactions in this introductory chemistry course from MSU Download the PDF lecture notes and practice with exercises and examples Explore how stoichiometry can be applied to real world problems such as environmental issues industrial processes Collectively these conversions are called mole mass calculations A stoichiometry calculation converting between masses and moles of different substances in a chemical reaction As an example consider the balanced chemical equation Fe 2 O 3 3SO 3 Fe 2 SO 4 3 If we have 3 59 mol of Fe 2 O 3 how many grams of SO 3 can react with it Using the mole mass calculation sequence we can

Erin sChemBlog Stoichiometry
http://3.bp.blogspot.com/-e43HN2zRfrM/VnCvNgDrFiI/AAAAAAAAAwA/MQV9DQewBso/s1600/Stoichiometry%2BMole%2BTunnel.png

Stoichiometry With Mass YouTube
https://i.ytimg.com/vi/C8S11U6VnBQ/maxresdefault.jpg
Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers - Learn how to use a balanced chemical equation to calculate the amounts of reactants and products involved in a chemical reaction This chapter explains the concepts and methods of reaction stoichiometry including mole mole mass mass and limiting reactant calculations Explore examples and exercises from the OpenStax Chemistry 2e textbook