Typical Drug Development Timeline May 12 2015 nbsp 0183 32 Its main focus is to aid new drug developers and companies developing unique products such as gene and cell therapy nanomedicines or treatments involving new delivery systems or produced through novel manufacturing processes
The development timeline generally includes several key phases drug discovery preclinical testing clinical trials and regulatory review Each of these stages is crucial for ensuring that a drug is safe and effective In general the drug development pipeline including preclinical development clinical research and major regulatory approvals can be summarized as follows 1 Drug discovery 2 Pre clinical research Preclinical development laboratory studies 3 Investigational New Drug IND application 4 Clinical trials 5 New Drug Application NDA
Typical Drug Development Timeline
Typical Drug Development Timeline
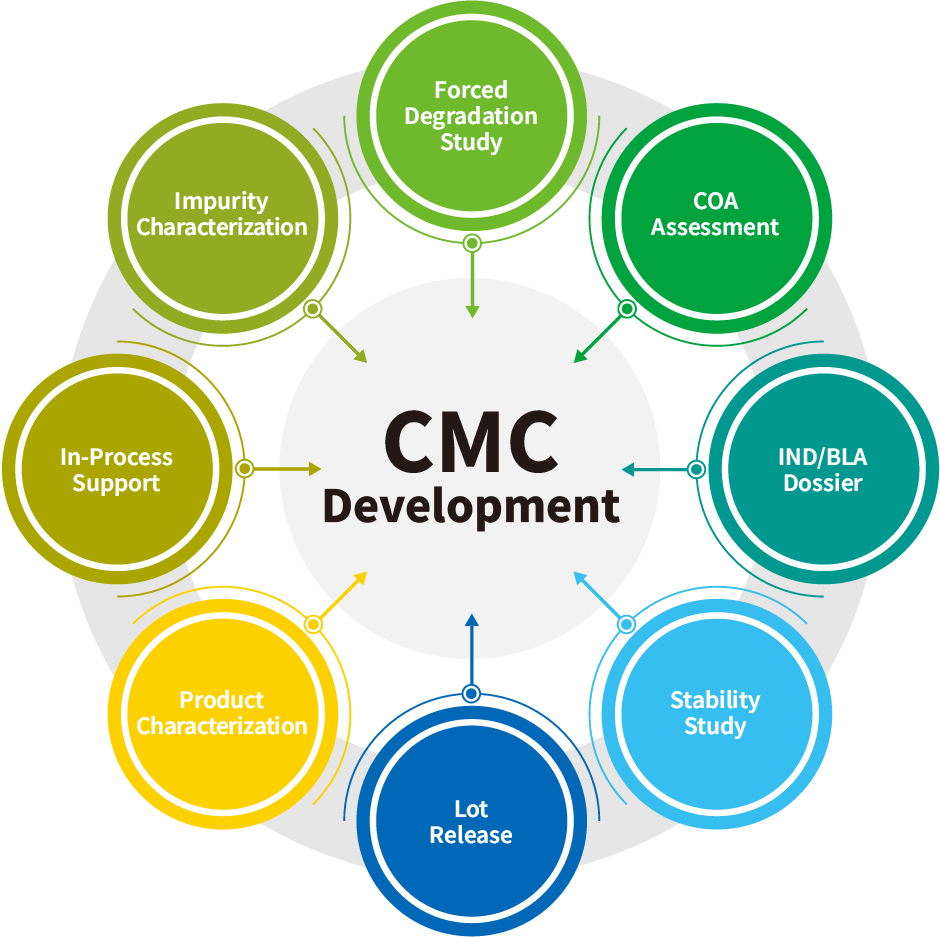
https://wuxivaccines.com/wp-content/uploads/2023/10/CMC-Development.png
V3 Characters Timeline Visualised 3 1 3 5 Based On Speculation From SYP
https://i.redd.it/et1yr758i0n91.jpg

Drake Grammy Wins
https://i8.amplience.net/i/naras/Drake_Grammy_Hero_1644x925.jpg
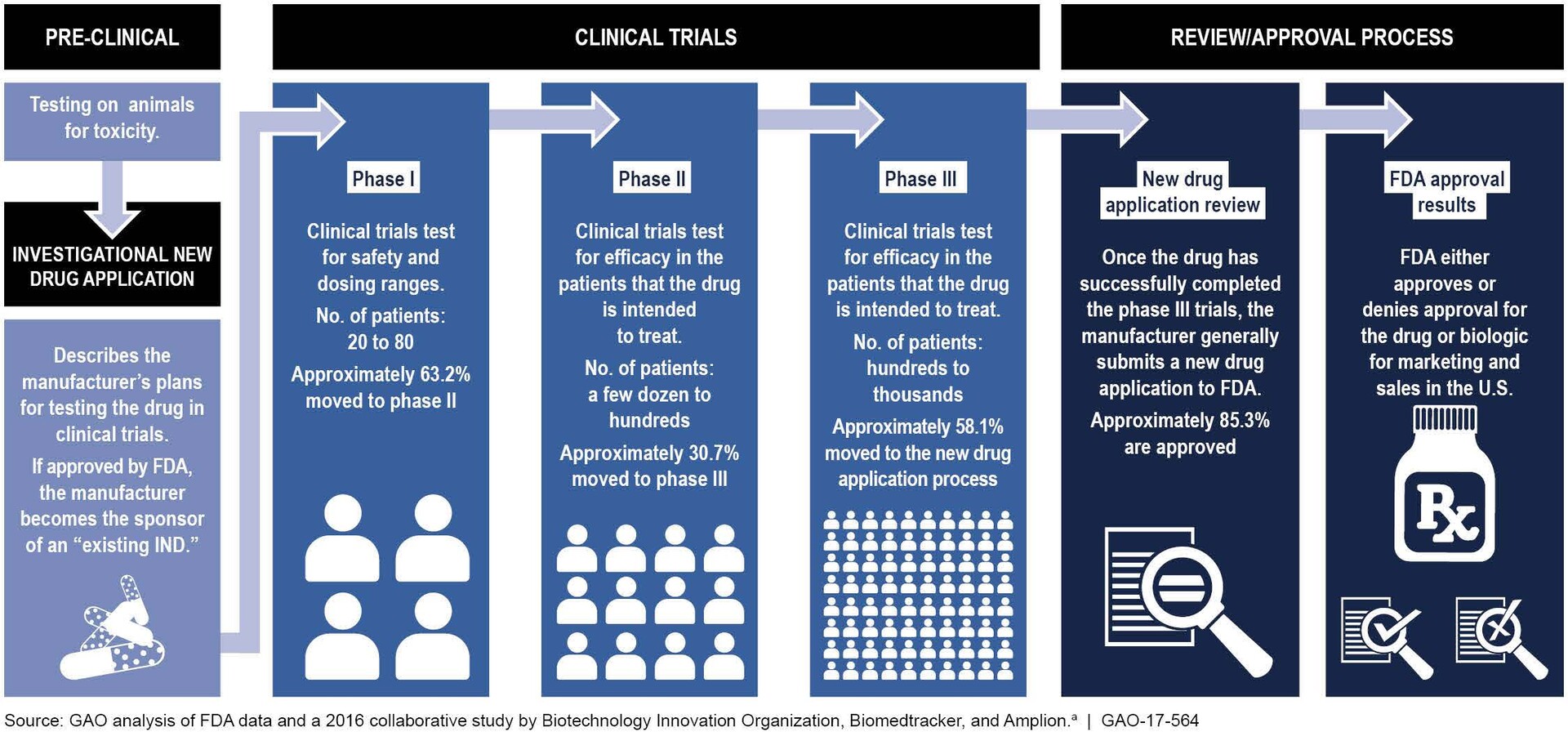
Feb 1 2023 nbsp 0183 32 The average lifecycle of drug development from discovery to approval is about 10 to 12 years The total time varies depending on the previous knowledge and experience gained by similarity to an approved and licensed drug product that is on the market and the type of regulatory approval process that the drug product goes through Figure 1 Drug development is typically subdivided into 4 main stages Drug Discovery Pre Clincal Research Clinical Development and Marke Approval Starting from thou sands of compounds being screened for target validation one drug is finally approved after 12 15 years
The process of getting a drug to market from first testing to final FDA approval is summarized in figure 1 and described at greater length below Drug companies continuously analyze thousands of compounds seeking ones of therapeutic value Apr 10 2025 nbsp 0183 32 The drug development timeline encompasses several key stages each vital for the comprehensive evaluation of new drugs Understanding these stages provides constructive insights for improving strategies and increasing the likelihood of successful drug approvals
More picture related to Typical Drug Development Timeline
Understanding Investigational Drugs StoryMD
https://cdn.storymd.com/optimized/Oo5gO3cDqK/original.jpg
Typical Traditional Nepali House Drawing Design Clipart Clipart Nepal
https://clipartnepal.com/wp-content/uploads/2023/12/Typical-traditional-nepali-house-drawing-design-clipart.jpg
Timeline APK For Android Download
https://images.sftcdn.net/images/t_app-cover-l,f_auto/p/ece785c9-4c85-4f7d-abe4-6296c50c3487/2680295597/timeline-screenshot.png
Understanding drug development timelines is essential in Pharma and Biotech Industry Management This process includes preclinical research clinical trials and regulatory approvals all aimed at ensuring new drugs are safe and effective before reaching the market Each phase plays a critical role Preclinical research phase A successful drug in the market has necessarily passed through five main stages that include Drug Discovery and development preclinical research clinical trials regulatory approval and post market monitoring
[desc-10] [desc-11]

Superman Selfie Wearing Blue Superman Costume With Red Cape Sunny
https://img-musesai.163264.com/pic/202406/MphKZox07mYk.jpg

Pin On Drug Development
https://i.pinimg.com/originals/40/0f/db/400fdb2218ee87978ced1efa318f6811.png
Typical Drug Development Timeline - The process of getting a drug to market from first testing to final FDA approval is summarized in figure 1 and described at greater length below Drug companies continuously analyze thousands of compounds seeking ones of therapeutic value