Types Of Intermolecular Forces Worksheet Chemical bonds e g covalent bonding are intramolecular forces which hold atoms together as molecules The forces that hold molecules together in the liquid and solid states are called intermolecular forces and are appreciably weaker Intermolecular forces IMF can be qualitatively ranked using Coulomb s Law
CH2Cl2 and CH2Cl2 Dipole Dipole 2 If the pairs of substances listed below were mixed together list the intermolecular force s that are involved Choices Hydrogen Bonding Standard Dipole Dipole London Forces induced dipole Ion Dipole Salt Bridges ionic forces Exercise x CHEM1101 Worksheet 7 Intermolecular Forces Information Intermolecular forces are the interactions between rather than inside molecules They are responsible for many of the physical properties of substances including their melting and boiling points In pure substances there are 3 important intermolecular forces which may be
Types Of Intermolecular Forces Worksheet
Types Of Intermolecular Forces Worksheet
https://imgv2-2-f.scribdassets.com/img/document/364102913/original/e6d7a8a3e1/1619061342?v=1
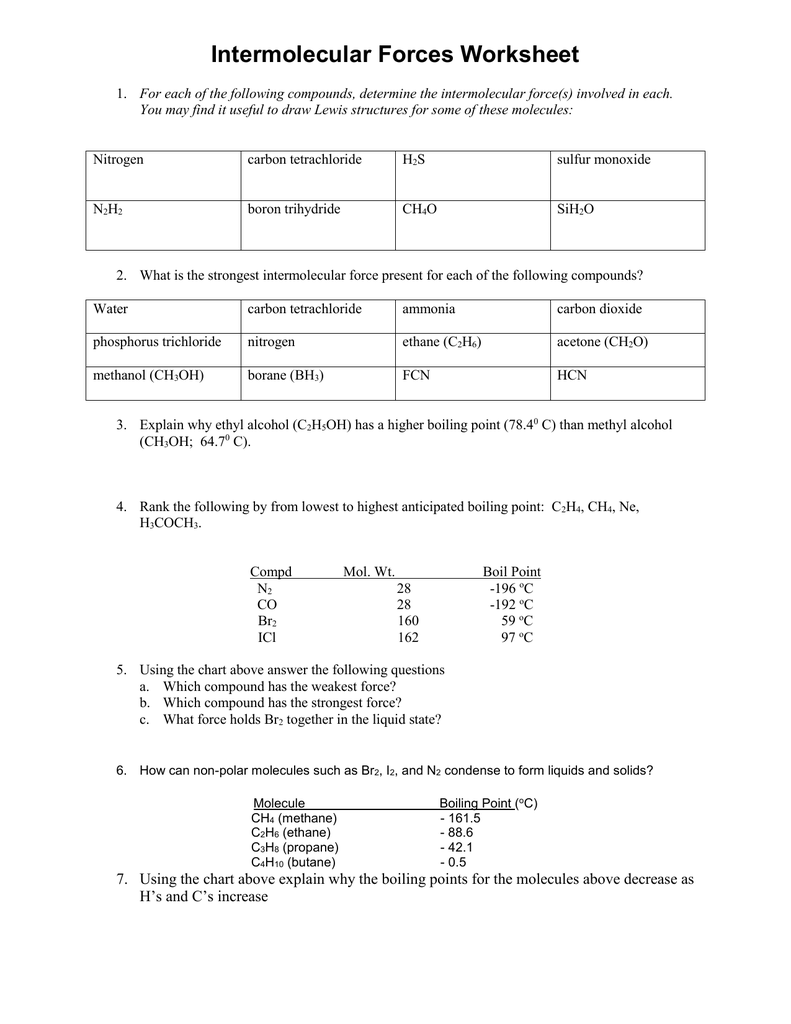
Intermolecular Forces Worksheet
https://s2.studylib.net/store/data/010014065_1-cd44f4ad6aea598aad59d21dd495cc87.png
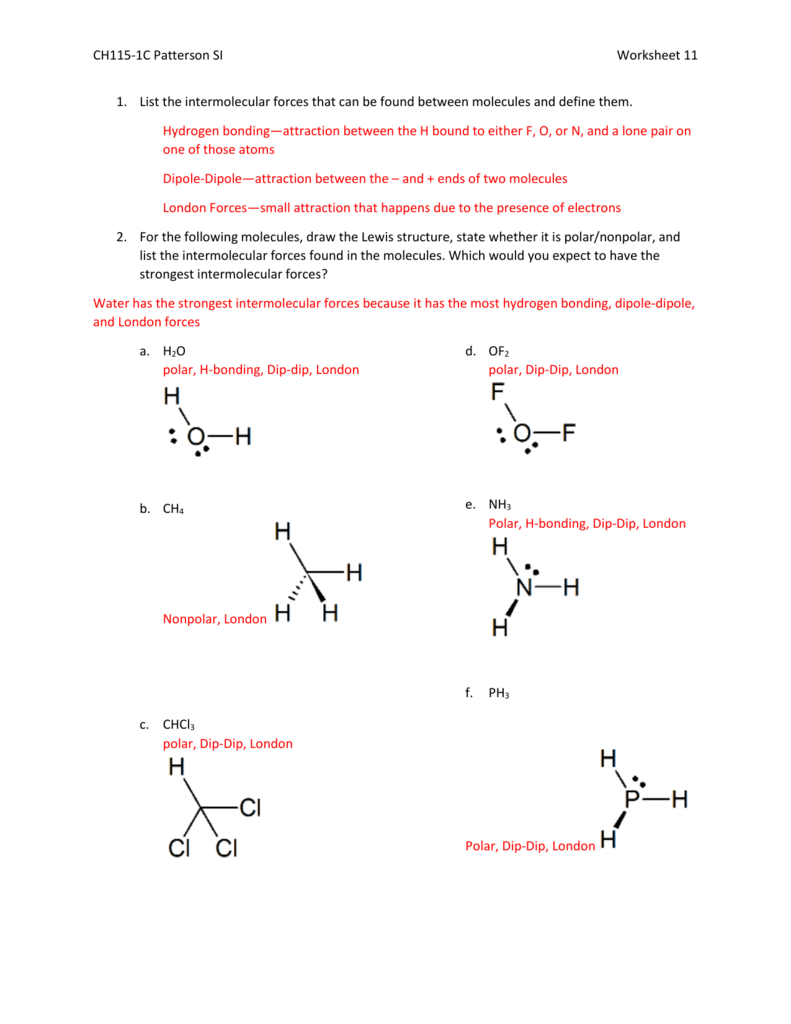
CH115 1C Patterson SI Worksheet 11 List The Intermolecular Forces
https://s3.studylib.net/store/data/007001649_1-cba62815f06a4839ac8a257287731914.png
Kinds of Intermolecular Forces We will consider the following types of intermolecular forces London dispersion dipole dipole and hydrogen bonding London dispersion forces and dipole dipole forces are collectively known as van der Waals forces Molecules can have any mix of these three kinds of intermolecular forces but all substances at 8 benzene C6H6 9 ammonia NH3 10 methanol CH3OH London dispersion forces London dispersion forces London dispersion forces dipole dipole forces hydrogen bonding dipole dipole forces
Intermolecular Forces Worksheet Obj 4 a g 1 Define each type of intermolecular force below 4b o Indicate the type of molecules involved polar or non polar o Indicate the relative strength of each o Give an example of a molecule that experiences each type from the video a Van der Waals forces b Dipole Dipole forces Molecules with more electrons experience stronger London dispersion forces In this simulation students will review the three major types of intermolecular forces London dispersion forces dipole dipole interactions and hydrogen bonding through short video clips and accompanying text They will then answer quiz questions using the
More picture related to Types Of Intermolecular Forces Worksheet

Quiz Worksheet Intermolecular Forces Physical Properties Study
https://study.com/academy/practice/quiz-worksheet-intermolecular-forces-physical-properties.jpg

Intermolecular Force Worksheet
https://s3.studylib.net/store/data/025201866_1-bed8e32b2c2ec7c6a077e29117ac3147.png

Intermolecular Forces Practice
https://s3.studylib.net/store/data/008550289_1-46905c53d7102fafec8272aa692f00bd-768x994.png
The intermolecular forces present explain the significant difference in the boiling points of these two substances 8 The boiling points of HCl HBr and HI are in order 85 C 67 C and 35 C a Name the two types of intermolecular forces between these HX molecules b Based on the trend in their boiling points which type of bonding Worksheet Intermolecular Forces 1 Draw Lewis structures for these molecules Use symbols to indicate bond dipoles Predict whether there is an overall molecular dipole List the dominant type of IMF for the pure substances then rank the strength of each compound based on IMFs within the samples 1 strongest 2 in between 3 weakest
Worksheet 15 Intermolecular Forces Chemical bonds are intramolecular forces which hold atoms together as molecules The forces that hold molecules together in the liquid and solid states are called intermolecular forces r2 where Q1 and Q2 are charges and r is the distance between them Types of Intermolecular Forces There are three types of intermolecular forces London dispersion forces LDF dipole dipole interactions and hydrogen bonding Molecules can have any mix of these three kinds of intermolecular forces but all substances at least have LDF

Intermolecular Forces
https://s3.studylib.net/store/data/025185963_1-7af5b3360a3618c6acae5e28e2e9d90c.png

Intermolecular Forces Worksheet Key covalen Bonds and Intermolecular
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/6a88db16d1620afd1a59acf6abeee7e8/thumb_1200_1553.png
Types Of Intermolecular Forces Worksheet - Kinds of Intermolecular Forces We will consider the following types of intermolecular forces London dispersion dipole dipole and hydrogen bonding London dispersion forces and dipole dipole forces are collectively known as van der Waals forces Molecules can have any mix of these three kinds of intermolecular forces but all substances at
