Pure Substance Vs Mixture Aug 23 2019 nbsp 0183 32 A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample Mixtures are physical combinations of two or more elements and or compounds
Feb 2 2017 nbsp 0183 32 The main difference between pure substance and mixture lies in their composition A pure substance contains only one kind of compound It can be the same molecule or atom Feb 6 2018 nbsp 0183 32 To put it simply pure substances are exactly what the name implies pure while mixtures are impure Summary 1 Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances 2
Pure Substance Vs Mixture
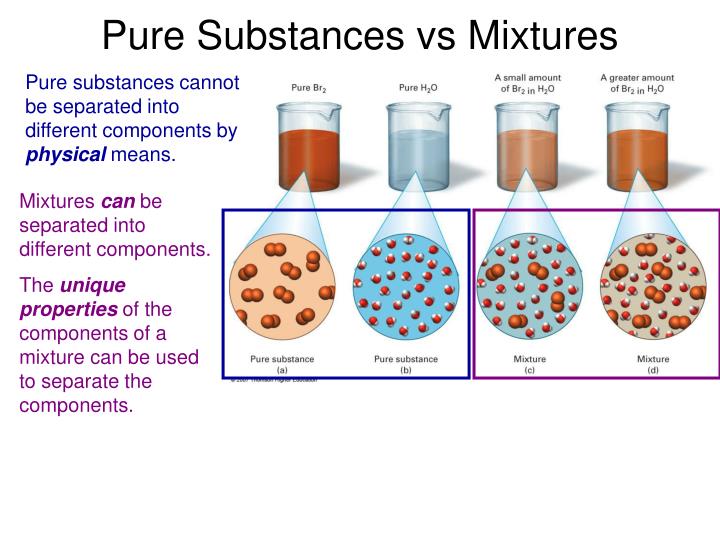
Pure Substance Vs Mixture
http://image2.slideserve.com/5074222/pure-substances-vs-mixtures-n.jpg
00 pure Substances Vs Mixtures Presentation
https://image.slidesharecdn.com/00-puresubstancesvs-mixturespresentation-120926125016-phpapp02/95/00pure-substances-vs-mixtures-presentation-1-1024.jpg?cb=1348663852
Pure Substance Vs Mixture Examples Slidesharedocs
https://lh4.googleusercontent.com/proxy/SG_7j7GdU7jofe85xqpvo2GhJOhAvlHlyqIYmHQwsLr7cHQPk6INglZQn7Ulmmj6qSLmQqUXNcQ_-3UAvqL5YHK1I4hbfiH9j91YcDHlbDHZu0D2vhdcr5Vy3y0=s0-d
Jul 21 2021 nbsp 0183 32 You can classify matter as a pure substance or as a mixture Learn the differences between the two and the different types of each Pure substances exhibit consistent physical and chemical properties allowing for predictable behavior under specific conditions On the other hand a mixture is a combination of two or more substances that are physically blended together
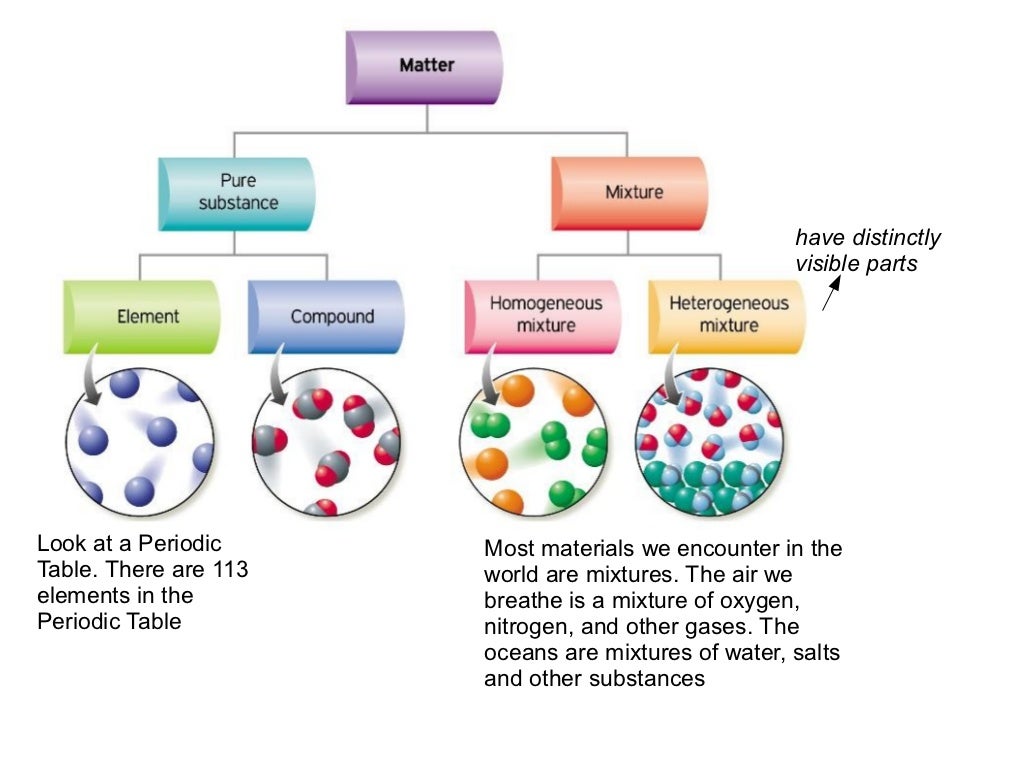
Sep 17 2024 nbsp 0183 32 Explain the difference between a pure substance and a mixture Explain the difference between an element and a compound Explain the difference between a homogeneous mixture and a heterogeneous mixture A pure substance is a substance made up of one component whereas a mixture is a substance made up of combining two or more elements The chemical and physical properties of the pure substance remain the same throughout but they change in a mixture
More picture related to Pure Substance Vs Mixture
Elements Compounds And Mixtures Differences Foto Kolekcija
https://lh4.googleusercontent.com/proxy/wxOKzFIzOJ4Fy0-bsMNGnYjOEG3xqcksExrzqVrvIuyqjdn87NcTqFVriDqRLQf7VqBhK8WXXdKrdxfQmoasgoz-mkR5-CeXxhRGdK2vHA=w1200-h630-p-k-no-nu

Chem Pure Substances Vs Mixtures YouTube
https://i.ytimg.com/vi/c74kJW4KoKA/maxresdefault.jpg

Pure Substances And Mixtures Classification Of Matter YouTube
https://i.ytimg.com/vi/pWZlICXw3Ng/maxresdefault.jpg
A sample of matter is one of two things it is either a pure substance that is a single substance or it is a mixture of two or more substances A pure substance contains only one type of substance Mixture and pure substance are two different types of matter A mixture is a combination of two or more substances that are physically combined and can be separated by physical means It does not have a fixed composition and can vary in its properties depending on the
[desc-10] [desc-11]

Difference Between Pure Substance And Mixture Definition Composition
http://pediaa.com/wp-content/uploads/2017/02/Difference-Between-Pure-Substance-and-Mixture-Comparison-Summary.png

00 pure Substances Vs Mixtures Presentation Teaching Materials
https://i.pinimg.com/originals/b3/e0/9f/b3e09fd80e8ec04cbcfc672b0f357988.jpg
Pure Substance Vs Mixture - Pure substances exhibit consistent physical and chemical properties allowing for predictable behavior under specific conditions On the other hand a mixture is a combination of two or more substances that are physically blended together