Persuasive Writing Lesson Plan Ks2 These documents serve to demonstrate the compliance of the investigator sponsor and monitor with the standards of Good Clinical Practice and with all applicable regulatory requirements
The requirements in this part apply to any applicant who submits a marketing application for a human drug biological product or device and who submits covered clinical studies FDFs are not a UK requirement but if a company is planning to submit a marketing application in another country such as the US they may be required to provide these forms as part of their
Persuasive Writing Lesson Plan Ks2
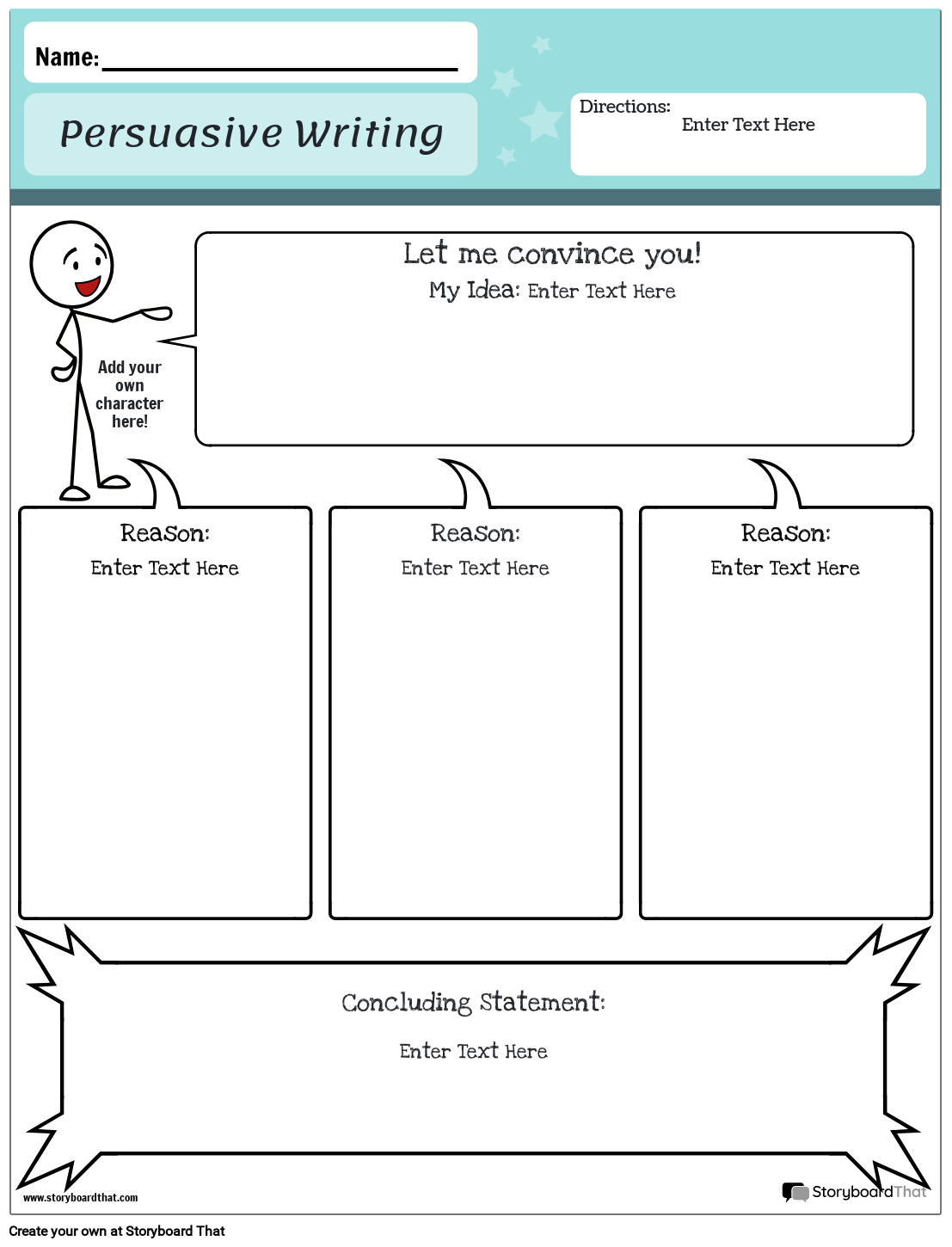
Persuasive Writing Lesson Plan Ks2
https://sbt.blob.core.windows.net/storyboards/worksheet-templates/persuasive-essay-worksheet-portrait-color-11.png
Story Structure Teaching Resources Vrogue co
https://d1e4pidl3fu268.cloudfront.net/7102b9d3-dec9-4618-8dca-973d0f22c681/Storymap.crop_711x533_0,0.preview.JPG

Detailed Lesson Plan On Persuasive Writing Pdf By Geostalubco1972 Issuu
https://assets.isu.pub/document-structure/230503103100-e69121bb4b9a896b3c14ff3441acd824/v1/75b17459fafcc96b103f05889822a367.jpeg
The objective of this ICH GCP Guideline is to provide a unified standard to facilitate the mutual acceptance of clinical trial data for ICH member countries and regions by applicable regulatory Feb 6 2020 nbsp 0183 32 FDA looks at several factors with regard to financial interest including the size and nature of the disclosed financial interest the steps taken to minimize the potential for bias and
When a clinical investigator has disclosable financial interests and arrangements the disclosure statement submitted to FDA is required to include a description of any steps taken to Jan 8 2019 nbsp 0183 32 A detailed description of FDA requirements for financial disclosure by clinical investigators The content includes 1 Five key definitions 2 Five significant penalties for
More picture related to Persuasive Writing Lesson Plan Ks2

Printable Writing Lesson Plan For Kindergarten PDF Included Number
https://numberdyslexia.com/wp-content/uploads/2023/03/Writing-lesson-plan-for-kindergarten-lesson-plan-1-768x1086.jpg

Persuasive Writing Lesson Plan Grade 5
https://i2.wp.com/lessonplanslearning.com/wp-content/uploads/2020/05/persuasive-letter-grade-4-writing-unit-4-pdf-free-download.jpg

Bbc Teach Macbeth Ks2 Songs And Worksheets
https://ichef.bbci.co.uk/images/ic/1008xn/p0572xvc.jpg
The applicant is required to submit for each clinical investigator who participates in a covered study either a certification that none of the financial arrangements described in 167 54 2 exist or Applicants that submit clinical data from covered clinical studies to FDA as part of a marketing appli cation for a device drug or biologic must comply with financial disclosure regulations in
[desc-10] [desc-11]
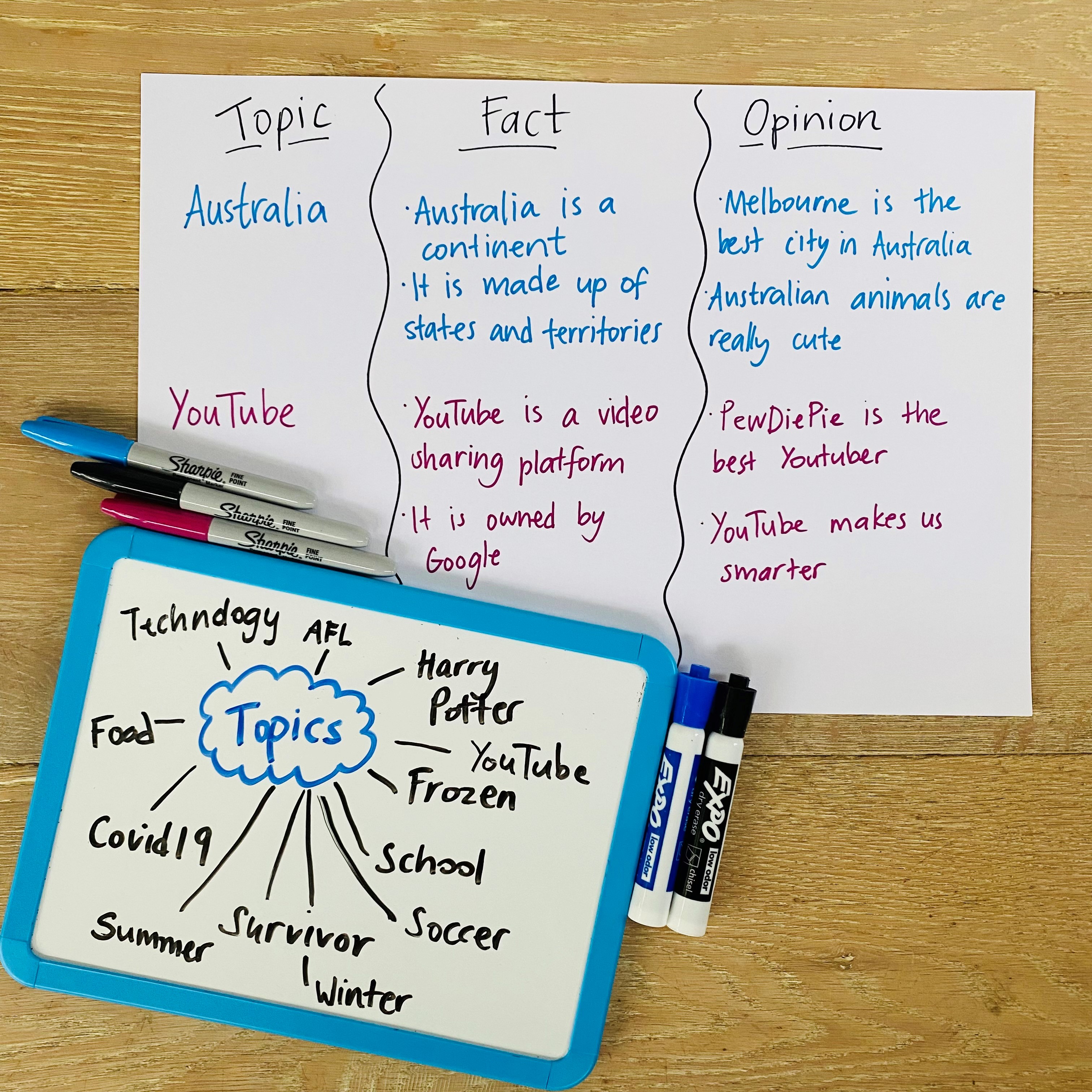
Persuasive Writing Cleverbean
https://images.ctfassets.net/ak9n2px9hdwx/2q6QlB3K1Phl5bOgyy8Wn7/12164f6ddd054c2b435095f67fc6dc04/CB2-BOOK-FNF-IMG-Annie-1723-Persuasive_Writing_-_Facts_and_Opinions-L1.jpg

Creative Writing Lesson Plans Ks2 Creative Writing In The Classroom
https://l.imgt.es/resource-preview-imgs/143dc744-e747-4afe-9c9f-c038aa0bde12/cover.crop_696x521_0,3.preview.jpg?profile%5Cu003dmax500x190
Persuasive Writing Lesson Plan Ks2 - Jan 8 2019 nbsp 0183 32 A detailed description of FDA requirements for financial disclosure by clinical investigators The content includes 1 Five key definitions 2 Five significant penalties for
