Periodic Trends Worksheet Atomic Radius Trends in sizes of atoms are the most important to understand because other trends can often be rationalized on that basis The most commonly used measure of size of an atom is its bonding atomic radius also called the covalent radius usually given in units of picometers pm 10 12 m or ngstroms 10 10 m with 1 100 pm
Explanation Periodic trends indicate that atomic radius increases up a group and from left to right across a period Therefore oxygen has a smaller atomic radius sulfur 10 Answer B False Explanation The reasoning behind this lies in the fact that a metal usually loses an electron in becoming an ion while a non metal gains an electron Electron electron repulsions due to their like charges electron pairs orient themselves as far away as possible from each other causing the electron cloud to expand justifies trends across a period 1 Atomic Radius Atomic radius increases Atomic radius is the distance from the atom s nucleus to the outer edge of the electron cloud
Periodic Trends Worksheet Atomic Radius
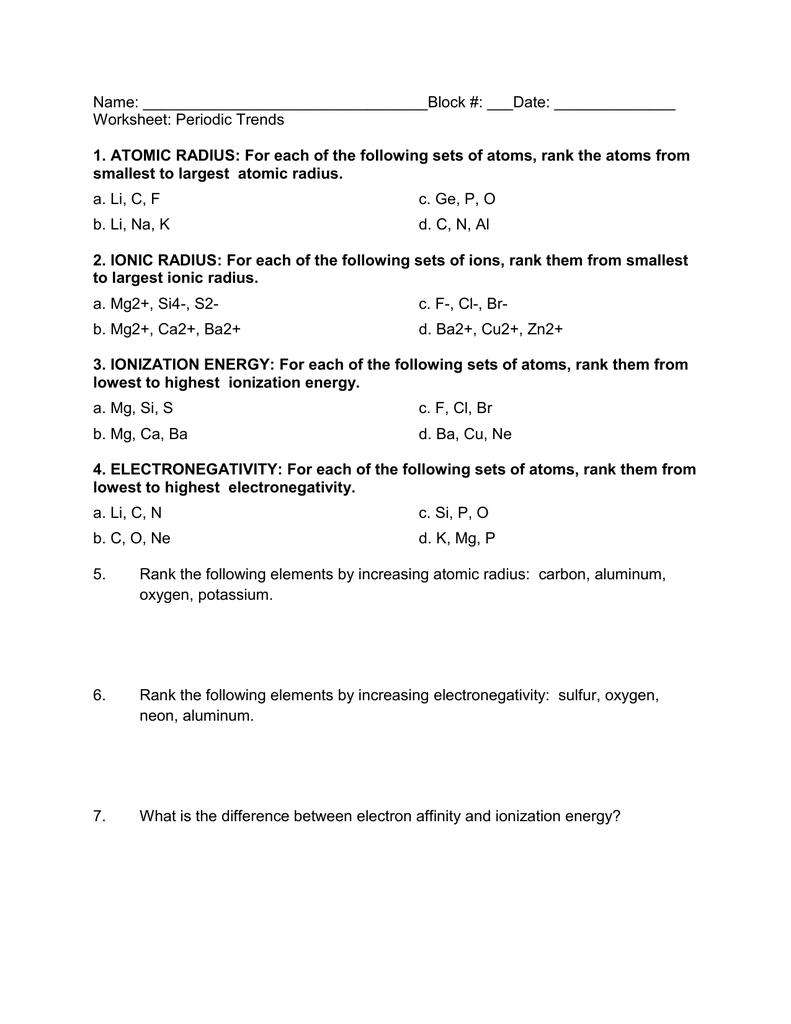
Periodic Trends Worksheet Atomic Radius
https://s2.studylib.net/store/data/009866398_1-a25910d39320271f6e572653b857205c.png
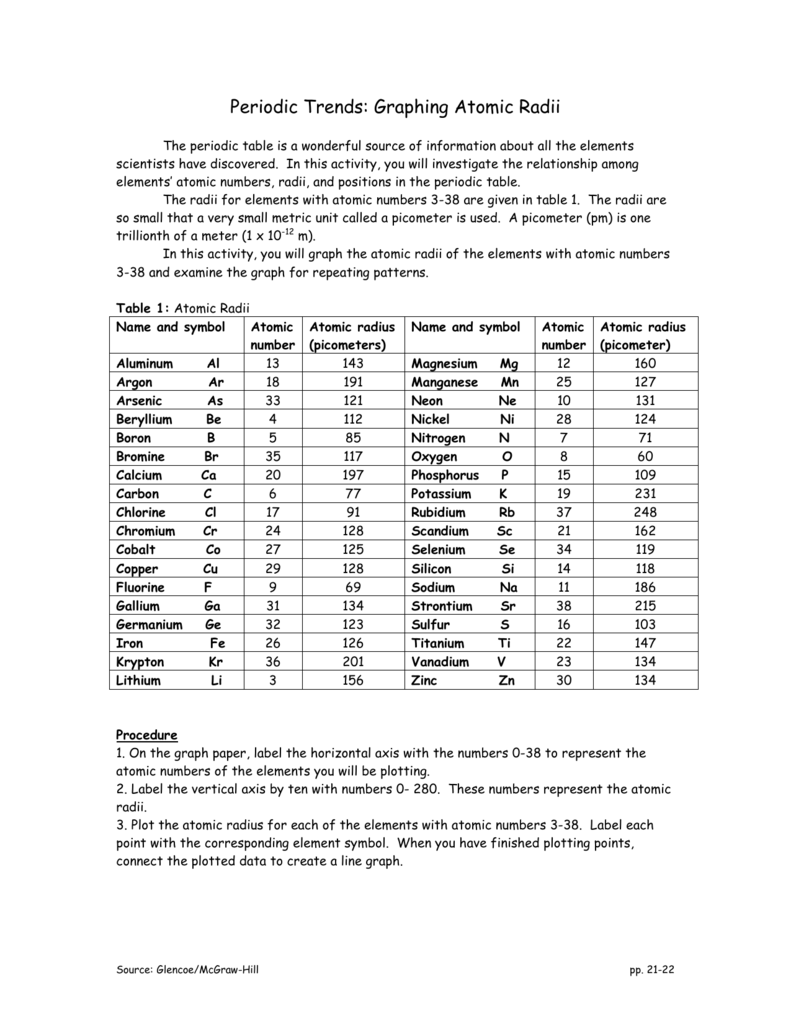
Periodic Trends Graphing Atomic Radii
https://s3.studylib.net/store/data/008850391_1-8ed9424341385a3fd030f9bf3a7570be.png

Periodic Table Trends Activity Teaching Chemistry Chemistry
https://i.pinimg.com/originals/f4/de/06/f4de0668d27c8856160efd6f2a717a6d.png
Jessica Martin Northeastern State University Trends in the Periodic Table I Atomic and Ionic Radius Worksheet is shared under a CC BY NC SA 4 0 license and was authored remixed and or curated by LibreTexts Many of the trends in the periodic table are useful tools for predicting electronic properties and chemical reactivities of various 5 Circle the atom in each pair that has the largest atomic radius a Al B b S O c Br Cl d Na Al e O F f Mg Ca 6 Put the following elements in order from smallest to largest atomic radius and explain why C O Sn Sr ELECTRONEGATIVITY
Worksheet Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C F b Li Na K d C N Al e Al Cl Ga Cli IONIC RADIUS For each of the following sets of ions rank them from smallest to largest ionic radius a Mg Si b Mg ca Ba c F cr Br d Ba cu Consider S Cl and K and their most common ions a List the atoms in order of increasing size Choose the larger atom in each pair a Al or In b Si or N c P or Pb d Si or Cl Choose the larger atom in each pair a Sn or Si b Br or Ga c Sn or Bi d Se or Sn In Table 7 8 the bonding atomic radius of neon is listed as 58 pm whereas
More picture related to Periodic Trends Worksheet Atomic Radius

1454015077 periodictrendsworksheet
https://s3.studylib.net/store/data/009635118_1-fe5c6c4667c60e36e43893d31353a87c.png

Worksheet Periodic Table Trends Answer Key 4 14 Brokeasshome
https://s1.studyres.com/store/data/000966540_1-df138a9992cb3d2189ffe4335f45797e.png
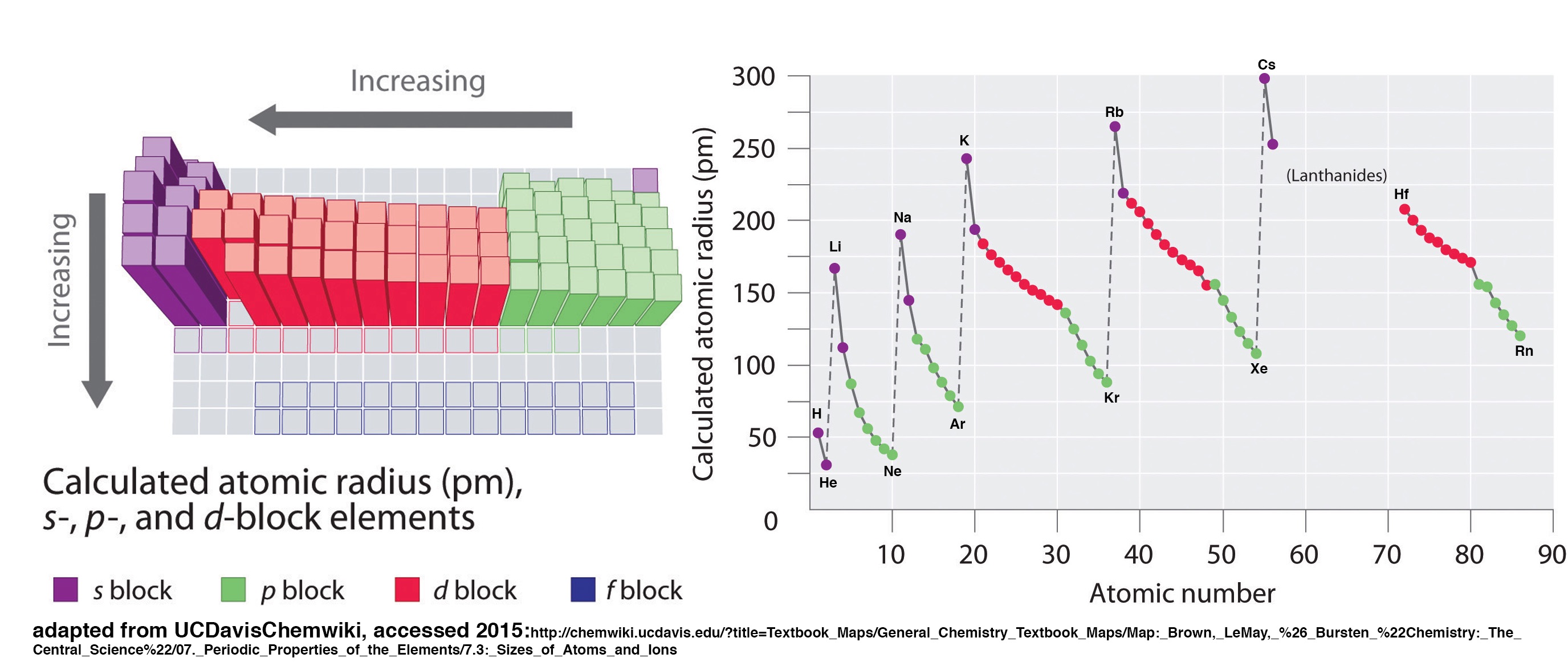
1 Periodic Trends Worksheet advanced Chemistry LibreTexts
https://chem.libretexts.org/@api/deki/files/240126/AtomicRadiusUCDavis.jpg?revision=1
Learn Periodic Trend Atomic Radius with free step by step video explanations and practice problems by experienced tutors Worksheet The Atom 0 Subatomic Particles 0 Isotopes 0 Ions 0 Based on the atomic radius trends of the periodic table sort the following elements from largest to smallest size Ar At Al The experimental Pb Cl bond length in lead II chloride PbCl2 is 244 pm Based on this value and data in Figure 7 7 predict the atomic radius of Pb Using only the periodic table arrange each set of atoms in order from largest to smallest c C Cl Cu Using only the periodic table arrange each set of atoms in order of increasing radius
The radius of an atom or its size Normally represented as covalent radii chemists determine this by measuring the distance between one element and another when bonded 3 Make a prediction about the following trends you expect to see in atomic radius a K P Br Na Fe 5 Indicate whether the following properties increase or decrease from left to right across the periodic table a atomic radius excluding noble gases b ionization energy c electronegativity Periodic Trends Worksheet 3 1 Rank the following elements by increasing atomic radius calcium iron neon nitrogen silicon

Atomic Radius Periodic Table NEET Lab
https://s3.amazonaws.com/neetlabcdn/wp-content/uploads/2017/11/29212947/atomic-radius-periodic-table.png

Understanding Atomic Radius Trends The 2 Key Principles
https://www.loyalmd.com/wp-content/uploads/sites/11/2017/07/pte.jpg
Periodic Trends Worksheet Atomic Radius - 4 Give your best and most concise explanation of the following trends a There is a general trend in atomic radius across the table it decreases as you go from left to right across a period b There is a general trend in atomic radius down the table it increases as you go down a group 5 Examine the charts above