Percent Composition Empirical And Molecular Formulas Worksheet Answers PROBLEM 4 4 1 10 4 4 1 10 Determine the number of moles of compound and the number of moles of each type of atom in each of the following a 25 0 g of propylene C 3 H 6 b 3 06 10 3 g of the amino acid glycine C 2 H 5 NO 2 c 25 lb of the herbicide Treflan C 13 H 16 N 2 O 4 F 1 lb 454 g
Solution To calculate the percent composition the masses of C H and O in a known mass of C 9 H 8 O 4 are needed It is convenient to consider 1 mol of C 9 H 8 O 4 and use its molar mass 180 159 g mole determined from the chemical formula to calculate the percentages of each of its elements C 9mol C molar mass C molar massC9H8O4 Therefore the subscripts moles in the empirical formula must be multiplied by two to obtain the molecular formula molecular formula 2 x empirical formula 2 x C 3 H 4 O 3 C 6 H 8 O 6 Calculate the molar mass of this formula to make sure it matches the one given in the problem M C 6 H 8 O 6 6 x 12 0 8 x 1 00 6 x 16 0 176 g
Percent Composition Empirical And Molecular Formulas Worksheet Answers

Percent Composition Empirical And Molecular Formulas Worksheet Answers
https://s3.studylib.net/store/data/006897671_1-e140165e4dd6212471727ac6bcd8a858.png

Percent Composition And Molecular Formula Worksheet
https://s3.studylib.net/store/data/007461692_1-1d0e0c491fb7c25b1c9f40e799b3b1a6.png

Empirical Formula And Molecular Formula From Percent Composition
https://i.ytimg.com/vi/xrkg9XtlFrI/maxresdefault.jpg
Percent Composition and Molecular Formula Worksheet 1 What s the empirical formula of a molecule containing 65 5 carbon 5 5 hydrogen and 29 0 oxygen 2 If the molar mass of the compound in problem 1 is 110 grams mole what s the molecular formula 3 What s the empirical formula of a molecule containing 18 7 lithium Calculate the empirical formula and the molecular formula of this compound given that the molar mass is 188 g mol 16 A compound contains 10 13 C and 89 87 Cl by mass Determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g mol 17 A certain compound has an empirical formula of
Chemistry 702 Percentage Composition and Empirical Formulas Chemistry A Study of Matter Semester 2 Instructions Before viewing an episode download and print the note taking guides worksheets and lab data sheets for that episode keeping the printed sheets in order by page number During the lesson watch and listen for instructions to By joining Chemistry Steps you will gain instant access to the Answers and Solutions for all the Practice Problems Quizzes and the powerful set of General Chemistry 1 and 2 Summary Study Guides 8 Calculate the percent composition by mass of the steroid hormone cortisol with the molecular formula of C 21 H 30 O 5
More picture related to Percent Composition Empirical And Molecular Formulas Worksheet Answers

SOLVED Empirical And Molecular Formula Worksheet Show ALL Your Work
https://cdn.numerade.com/ask_images/f6174d168d994b74a65b202a4714957d.jpg

Calculating Percent Composition And Empirical Formulas YouTube
https://i.ytimg.com/vi/mdNYDMoQ6As/maxresdefault.jpg
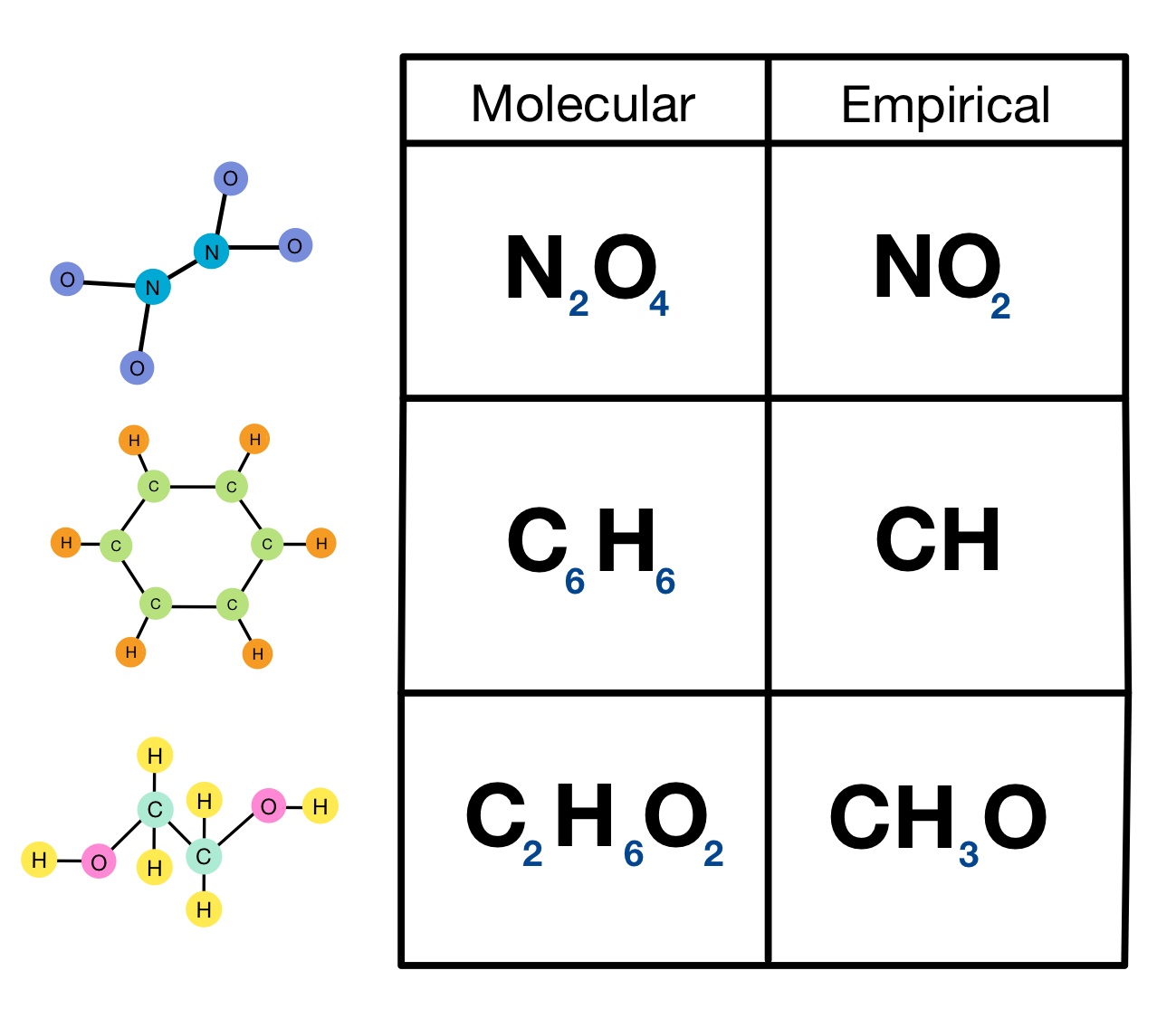
Empirical Formulas Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/empirical_formula_examples.jpeg
It includes 5 questions The second worksheet asks students to predict empirical and molecular formulas from given information It s interesting informative excellent practice This product comes in WORD format so it s easily edited to meet your needs Both worksheets include detailed answer keys Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
Molecular Formulas 5 The molar mass of the oxide of nitrogen in question 9 is 92 g mol Determine the empirical formula for each compound whose percentage composition is shown below 1 43 C and 57 O 2 40 3 K 26 7 Cr and 33 0 O Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps Principles of Chemistry Molecular and Empirical Formulas Practice Quiz What is the empirical and molecular formula for sucralose The percent composition is 36 25 C 4 82 H 26 75 Cl and 32 19 O The molar mass is 397 63 g mole a

Empirical And Molecular Formula Practice Solutions Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/f27b8372eab457d749d70fed0e1dc877.png?v=1646976324

Percent Composition Empirical Formula And Molecular Formula YouTube
https://i.ytimg.com/vi/CRzUbiESVAs/maxresdefault.jpg
Percent Composition Empirical And Molecular Formulas Worksheet Answers - To calculate the percent composition we need to know the masses of C H and O in a known mass of C 9 H 8 O 4 It is convenient to consider 1 mol of C 9 H 8 O 4 and use its molar mass 180 159 g mole determined from the chemical formula to calculate the percentages of each of its elements C 9molC molarmassC molarmassC 9H 18O 4 100