One Atomic Mass Unit Is Equal To What Dec 7 2015 nbsp 0183 32 1 atomic mass unit a m u is the mass of a proton or a neutron which is equal to 1 6726219xx10 27 kg The mass of a Hydrogen atom is 1 a m u But also we can say that
Feb 29 2024 nbsp 0183 32 One atomic mass unit a m u or amu is defined as one twelfth 1 12 of the mass of a carbon 12 atom which is approximately 1 660539040 215 10 27 kilograms It is a unit of An atomic mass unit AMU is a unit of mass used to express atomic and molecular weights It is defined as one twelfth 1 12 of the mass of a carbon 12 atom which is approximately equal to
One Atomic Mass Unit Is Equal To What

One Atomic Mass Unit Is Equal To What
https://i.pinimg.com/736x/8c/ff/a7/8cffa72d1763099f2aed24d53070fd6f--atomic-mass-unit-atomic-theory.jpg

Define The Atomic Mass Unit Teachoo Science Questions From Inside
https://d1avenlh0i1xmr.cloudfront.net/def633e1-2fc7-4617-8c05-ded336096bc5/atomic-mass-unit--01.jpg
Periodic Table Showing Mass Number And Atomic Number Periodic Table
https://lh6.googleusercontent.com/proxy/ny7XAYpKTmGy6gS-X0WTb-cIvOsSvWgOySAlKSW_bDmFZzRPYMteRp6lIPls_M5sARSfJAxYr-7JeQM1QkhMRJ9obm7MfsS2MgQk4thqNQiZjw4Swg-RiB3ElV8iTMDjs5pzysAzROc=s0-d
The atomic mass unit u or amu is a relative unit based on a carbon 12 atom with six protons and six neutrons which is assigned an exact value of 12 amu s An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon 12 The mass of any isotope of any element is expressed in relation to the carbon 12 standard For
One AMU is the sum of the proton and neutron rest masses in imprecise words 1 67377 x 10 27 kilogramme kg or 1 67377 x 10 24 gramme g In AMU an atom s mass is generally equal to Jun 30 2019 nbsp 0183 32 In chemistry an atomic mass unit or AMU is a physical constant equal to one twelfth of the mass of an unbound atom of carbon 12 It is a unit of mass used to express atomic masses and molecular masses
More picture related to One Atomic Mass Unit Is Equal To What
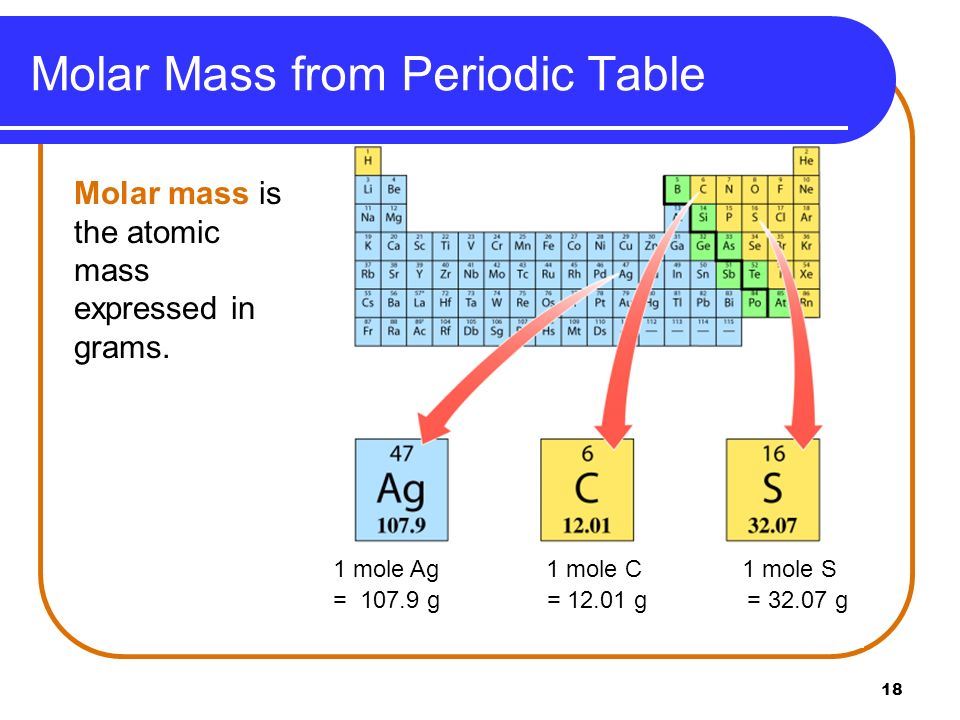
Atomic Mass Unit Metalxoler
https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/slide_18.jpg
Atomic Number Mass Number And Atomic Mass Unit
https://1.bp.blogspot.com/-abwBScahbY0/XqHq-LB4l1I/AAAAAAAAAEM/8oatJMgnADclwSu47mvmC2T8hptuKu8dACLcBGAsYHQ/s1600/2.png
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Difference Between Atomic Mass And Mass Number
https://www.thoughtco.com/thmb/l1lQtVgm--EbHJmFwCWzU0L84uU=/1500x1000/filters:fill(auto,1)/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png
The Atomic Mass Unit amu is a unit derived from the structure of the atom one amu atomic mass unit is exactly 1 12 th of the mass of one carbon 12 atom 1 amu 1 6605 215 10 24 g Atomic mass is expressed as a multiple of one twelfth the mass of the carbon 12 atom 1 992646547 215 10 23 gram which is assigned an atomic mass of 12 units In this scale 1 atomic mass unit amu corresponds to 1 660539040 215 10 24
The atomic mass unit u or amu is one unit for measuring the atomic mass of particles It is defined as one twelfth 1 12 of the mass of an unbonded Carbon 12 This is equal to Nov 12 2023 nbsp 0183 32 A unified atomic mass unit u is a standard unit of measurement used in biology to express the mass of atoms and molecules where one u is approximately equal to the mass of
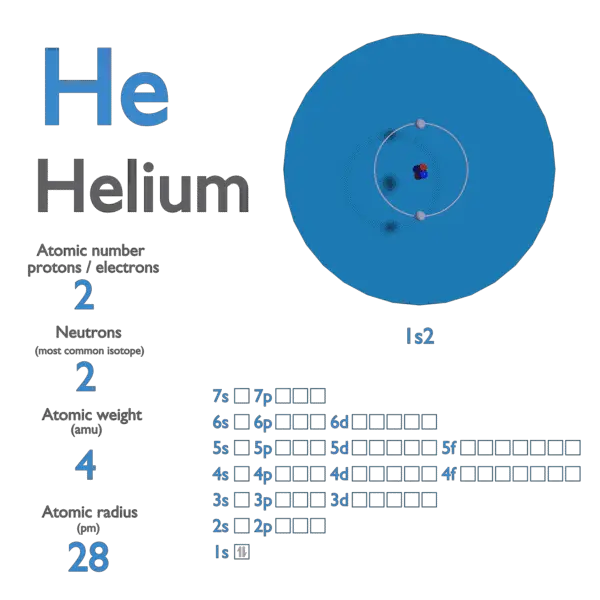
Helium Atomic Number Atomic Mass Density Of Helium Nuclear
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Helium.png

The Energy Equivalent Of One Atomic Mass Unit Is YouTube
https://i.ytimg.com/vi/GQo5I-D1B1k/maxresdefault.jpg
One Atomic Mass Unit Is Equal To What - The atomic mass unit u or amu is a relative unit based on a carbon 12 atom with six protons and six neutrons which is assigned an exact value of 12 amu s