Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions Nomenclature Worksheet 1 Monatomic Ions Use a periodic table to complete the table below Ion Formula Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions Please complete the following table Nomenclature Worksheet 5 Ionic Compounds Summary Name the following compounds
Oxyanions You may have noticed that most of the polyatomic ions in Table 5 7 1 5 7 1 carry a negative charge and contain the element oxygen Anions that contain the element oxygen are called oxyanions Many oxyanions belong to a series where the number of oxygen atoms in the ion varies as shown in Table 5 7 2 5 7 2 Sulfite SO 3 2 dichromate Cr 2 O 7 2 triiodide I 3 The naming of ionic compounds that contain polyatomic ions follows the same rules as the naming for other ionic compounds simply combine the name of the cation and the name of the anion Do not use numerical prefixes in the name if there is more than one polyatomic ion the
Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions

Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions
https://briefencounters.ca/wp-content/uploads/2018/11/charges-of-ions-worksheet-answers-with-polyatomic-ions-worksheet-sample-free-download-of-charges-of-ions-worksheet-answers.png

Common Polyatomic Ions Names Formulae And Charges Compound Interest
https://i2.wp.com/www.compoundchem.com/wp-content/uploads/2016/05/Guide-to-Common-Polyatomic-Ions.png?w=1488&ssl=1
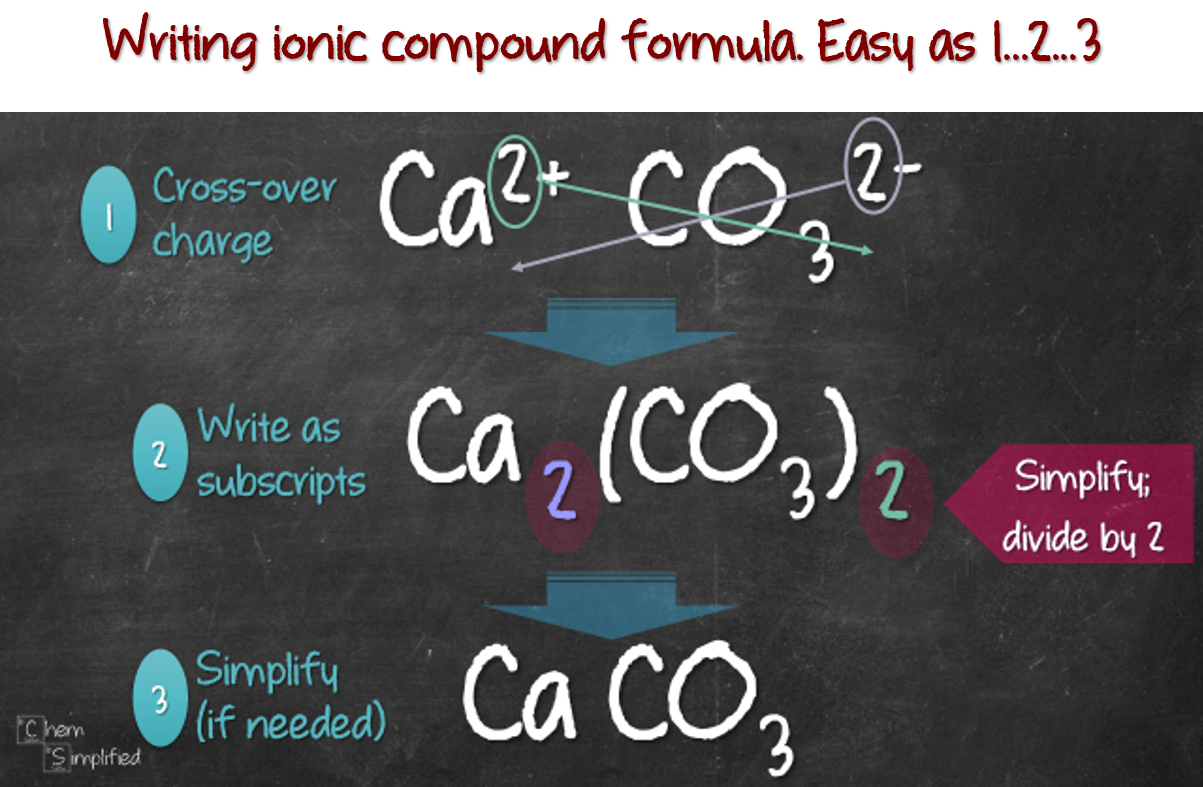
Writing Formula For Ionic Compounds ChemSimplified
https://chemsimplified.com/wp-content/uploads/2018/04/Writing-formula-for-ionic-compound.png
Question Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic lons Please complete the following table Name of lonic Compound Formula of lonic Compound 1 Sodium chromate 2 Calcium carbonate 3 Magnesium nitrate 4 Aluminum sulfate 5 Lithium phosphate 6 Ammonium chloride 7 Cesium chlorate 8 Potassium sulfate 9 Barium acetate 10 Polyatomic ions Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit but unlike molecules they have a net charge on them The examples include cations like ammonium ion NH 4 NH 4 and hydronium ion H3O H 3 O and anions like hydroxide ion OH OH and
Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions Please complete the following table Formula of Ionic Compound 2 3 4 5 Rules for Naming Ionic Compounds Containing Polyatomic Ions Polyatomic ions are ions which consist of more than one atom For example nitrate ion NO 3 contains one nitrogen atom and three oxygen atoms The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit
More picture related to Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions
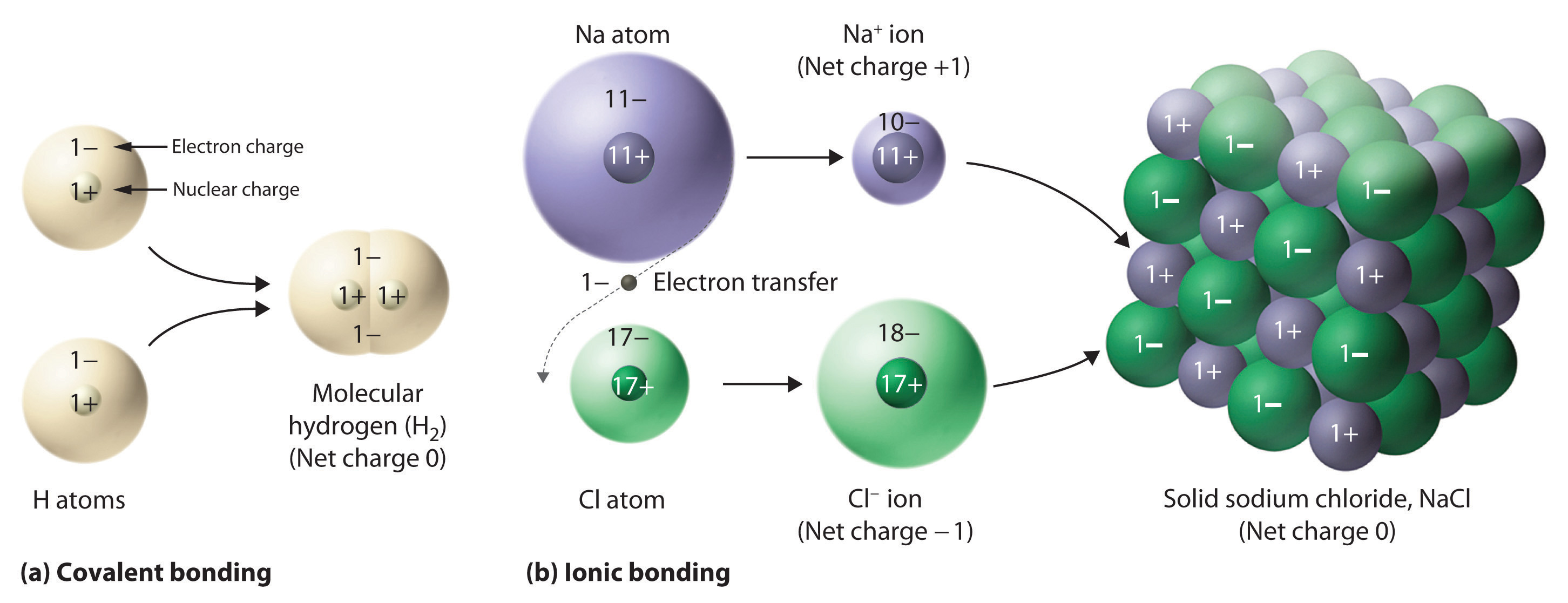
2 7 Ions And Ionic Compounds Chemistry LibreTexts
https://chem.libretexts.org/@api/deki/files/30371/4b1092559c38c43dd6fe074d07ee63b6.jpg?revision=3

What Are Polyatomic Ions Give Examples Teachoo Concepts
https://d1avenlh0i1xmr.cloudfront.net/5776d602-6722-415c-988c-d83ab9a5bb9b/examples-of-polyatomic-ions-teachoo-01.jpg
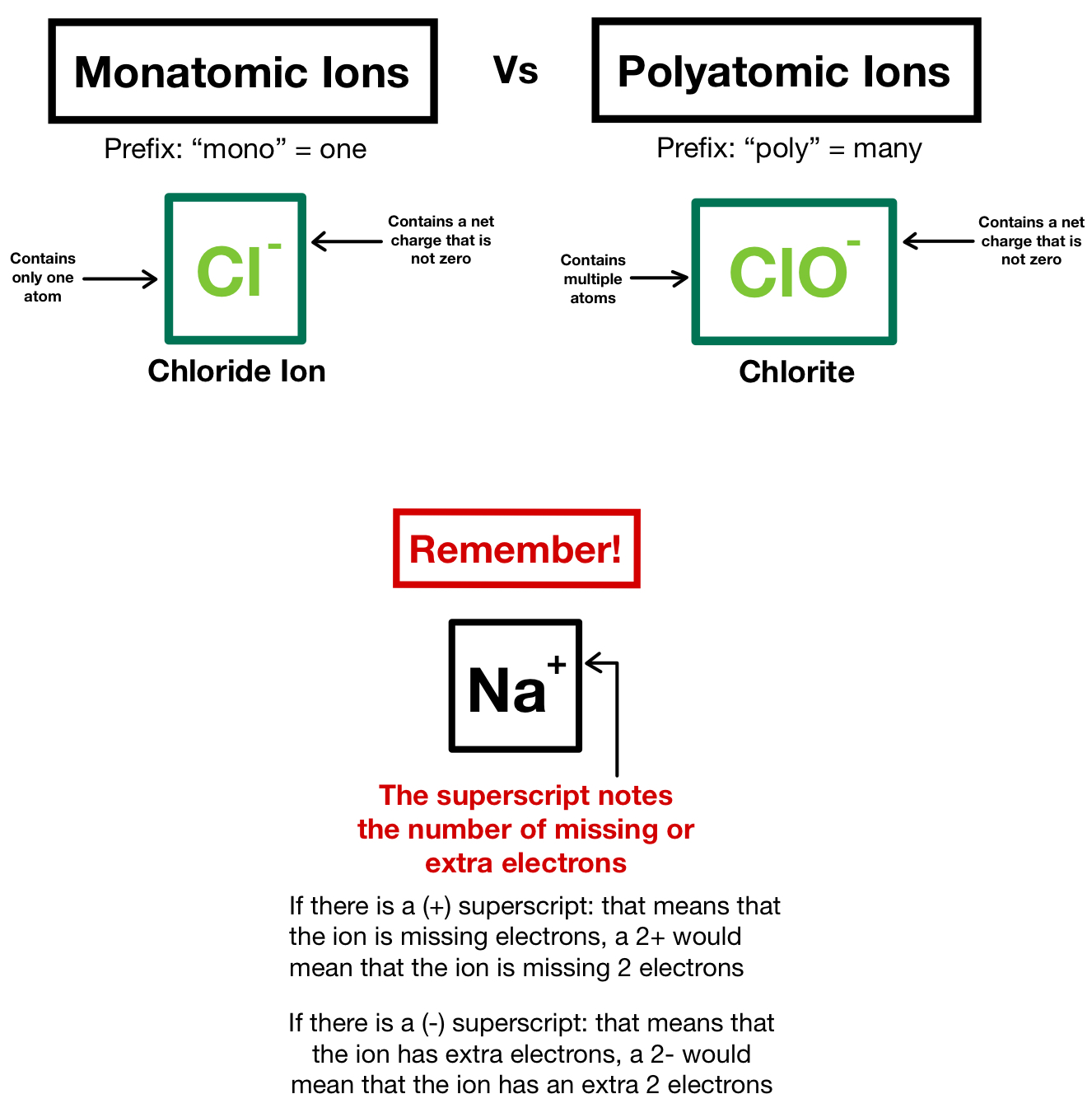
Polyatomic Ions Nomenclature Compounds Expii
https://d20khd7ddkh5ls.cloudfront.net/monatomic_ions_vs_polyatomic_ions.jpeg
Polyatomic ions Polyatomic ions are charged groups of atoms An example is ammonium ion NH 4 It has five atoms one nitrogen and four hydrogens that share a charge of 1 The polyatomic ions remain intact and parentheses may be required when using subscripts For example ammonium chloride is NH 4 Cl and ammonium sulfide is NH 4 2 S Dr Scott Beaver Page 3 of 6 Exercise 1 Complete the table of neutral ionic compounds with the formulas and names for each cation anion pair Nomenclature for polyatomic ions Worksheet Answer Key SO 4 2 NO 3 PO 4 3 CO 3 2 ClO 3 OH Na Na 2 SO 4 sodium sulfate NaNO 3 sodium nitrate Na 3 PO 4 sodium phosphate Na 2 CO 3 sodium carbonate NaClO 3
Naming Ionic Compounds Nomenclature Rules When naming ionic compounds list the cation first and the anion second The cation is the element name followed by a Roman numeral in parentheses if the element has multiple charges The anion has the ide ending for a binary compound or else a polyatomic ion name Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class education for anyone anywhere

Naming Ionic Compounds Nomenclature Rules Classwork And Homework
https://home.storage/9b45a995/https/35447f/sciencenotes.org/wp-content/uploads/2021/10/Naming-Ionic-Compounds.png

How To Memorize Polyatomic Ions Chemical Formulas SuperHuman Academy
https://superhumanacademy.com/wp-content/uploads/2018/08/Naming-polyatomic-ions.png
Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions - Polyatomic ions Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit but unlike molecules they have a net charge on them The examples include cations like ammonium ion NH 4 NH 4 and hydronium ion H3O H 3 O and anions like hydroxide ion OH OH and