Molarity And Molality Practice Problems Worksheet Answers 5 Molarity is affected by changes in pressure while molality is unaffected by changes in pressure 6 Molarity may result in an imprecise and inaccurate concentration while molality results in an
Define Molarity Calculate the molarity of a solution containing 5 g of N aOH in 500 ml of solution Molarity is the number of moles of the solute dissolved per liter of the solution Thus M mol per L All mole calculations will determine the amount in moles of the solution for which it is the
Molarity And Molality Practice Problems Worksheet Answers

Molarity And Molality Practice Problems Worksheet Answers
https://i2.wp.com/s3.studylib.net/store/data/008884677_1-ff7d48d968821ae21517a2feda97d705.png

Molarity Molality And Normailty Honors Chemistry Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/d8061cc664632982436580c6abea7a87/thumb_1200_1553.png

Molarity Practice Problems Worksheet
https://i.ytimg.com/vi/t1ljg3adZW8/maxresdefault.jpg
Click here to get an answer to your question molarity of 720 gm of pure water is Molarity can be directly calculated from strength if density is known by weight x 10xd Molarity GMM where d is density and GMM is gram molecular mass
So the molarity is function of volume which can be affected by changing the temperature of system while the molality of system is function of mass which does not have any effect of Molality may be a measure of the concentration of a solute during a solution in terms of the quantity of substance during a specified amount of mass of the solvent Formation of term
More picture related to Molarity And Molality Practice Problems Worksheet Answers
Molarity And Molality Practice Problems Concentraci n Molar Soluci n
https://imgv2-2-f.scribdassets.com/img/document/141358172/original/240eb9ba79/1567094325?v=1

Molar Ratio Practice Problems Answer Key
https://i.pinimg.com/736x/00/8a/b6/008ab6cdfa7b614a6696ac286fa7c640.jpg

Practice Molarity And Molality
https://image.slidesharecdn.com/practice-molarityandmolality-131209165138-phpapp02/95/practice-molarity-and-molality-1-638.jpg?cb=1386626442
The molarity and molality of a solution are M and m respectively if the molecular weight of the solute is M calculate the density of the solution in terms of M m and M Extensive properties depend on the quantity of matter but intensive properties do not Explain whether the following properties are extensive or intensive Mass internal energy pressure
[desc-10] [desc-11]

Molarity Practice Problems YouTube
https://i.ytimg.com/vi/o_iETsDSvkg/maxresdefault.jpg
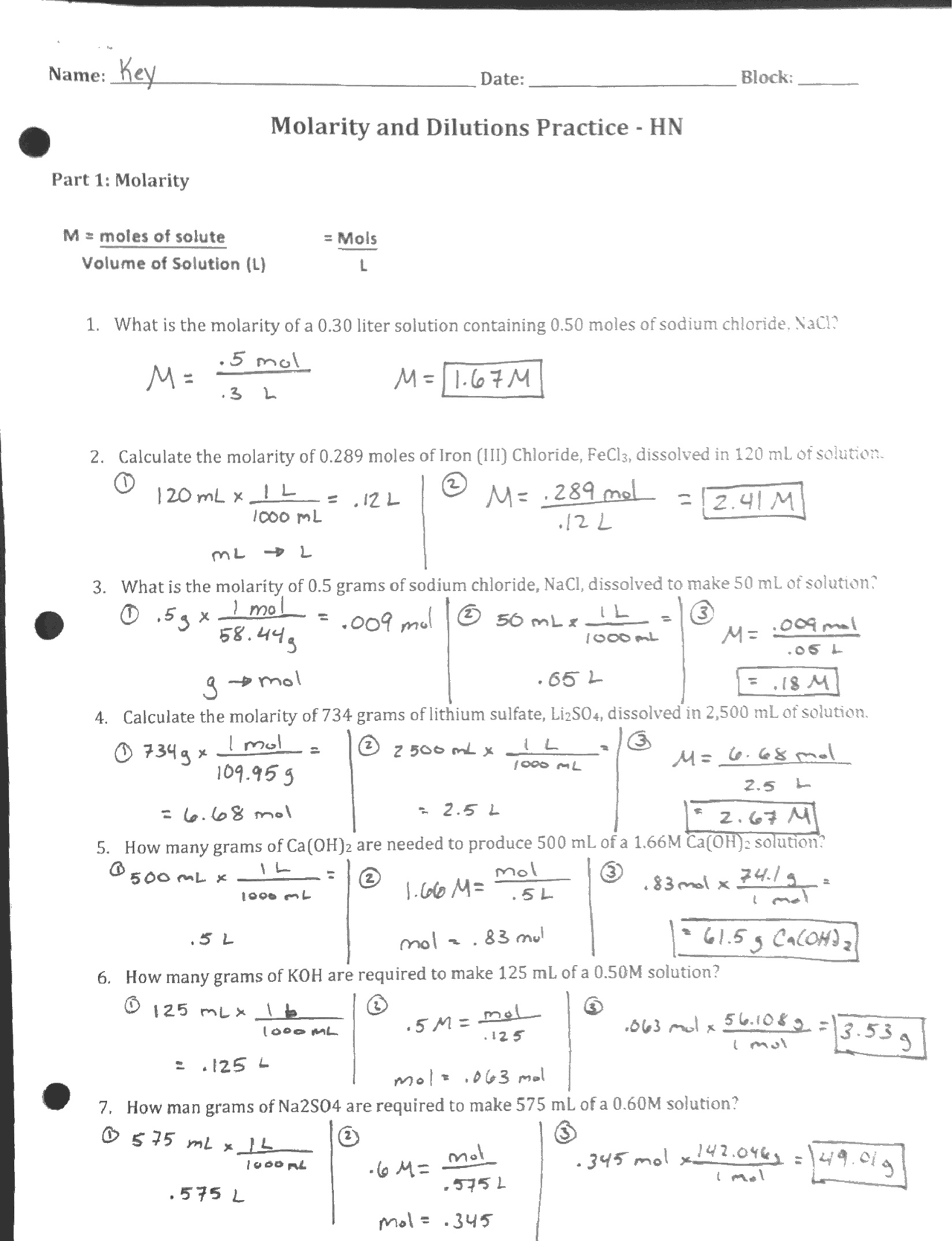
Molarity And Dilutions Practice Worksheet Key Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/6001daadf7683f72f8dab401484a4713.png?v=1651987055
Molarity And Molality Practice Problems Worksheet Answers - [desc-12]
