Mass Of Sodium Is 23 So How Many Protons And Neutrons Present In The Atom Sodium Na has an atomic number of 11 which means it has 11 protons The atomic mass of an atom is the sum of its protons and neutrons Therefore if the atomic mass of sodium is 23
May 16 2018 nbsp 0183 32 Mass Number The mass number of sodium is 23 The mass number is the total number of protons and neutrons in the nucleus To find the number of neutrons we subtract Mar 25 2024 nbsp 0183 32 The mass number is the sum of protons and neutrons so with a mass number of 23 and 11 protons there are 12 neutrons in the nucleus of the sodium atom When the sodium
Mass Of Sodium Is 23 So How Many Protons And Neutrons Present In The Atom
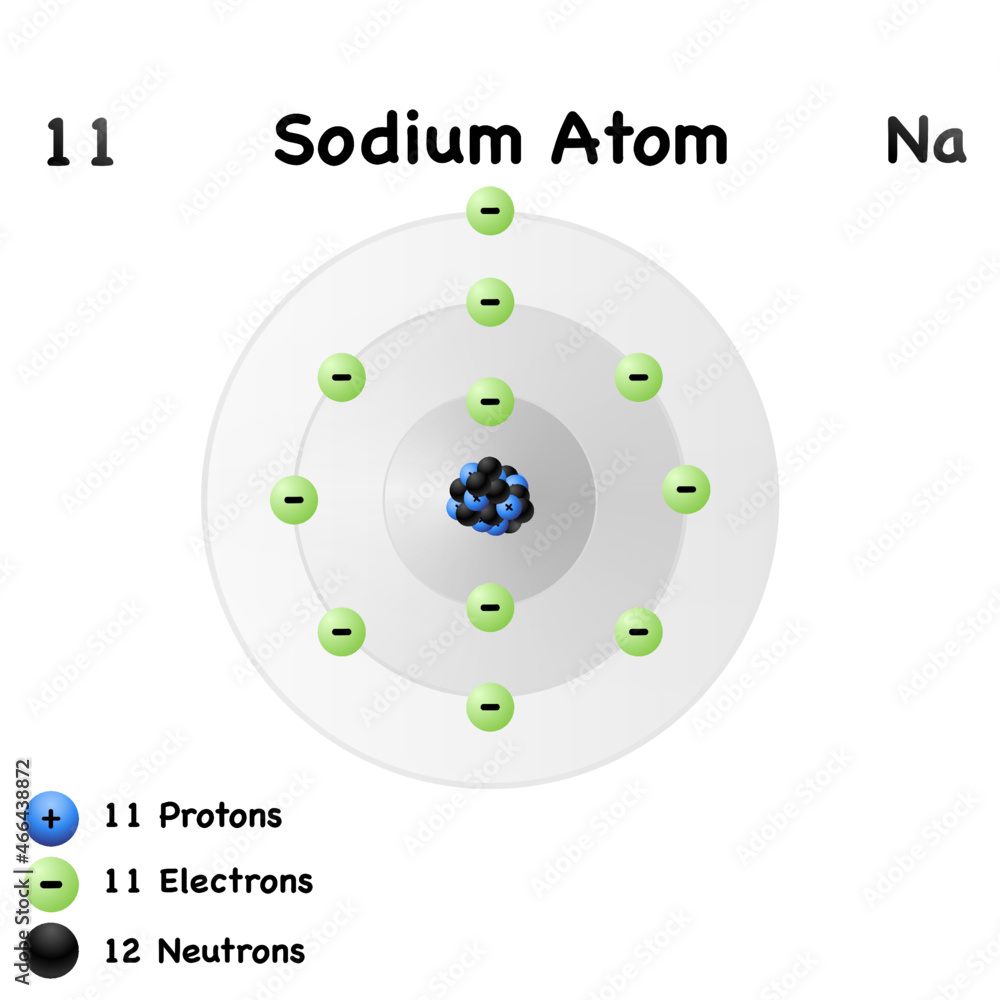
Mass Of Sodium Is 23 So How Many Protons And Neutrons Present In The Atom
https://as1.ftcdn.net/v2/jpg/04/66/43/88/1000_F_466438872_SN7nIzOC32sOPKpuAtxQPxJpwpDbIXiF.jpg
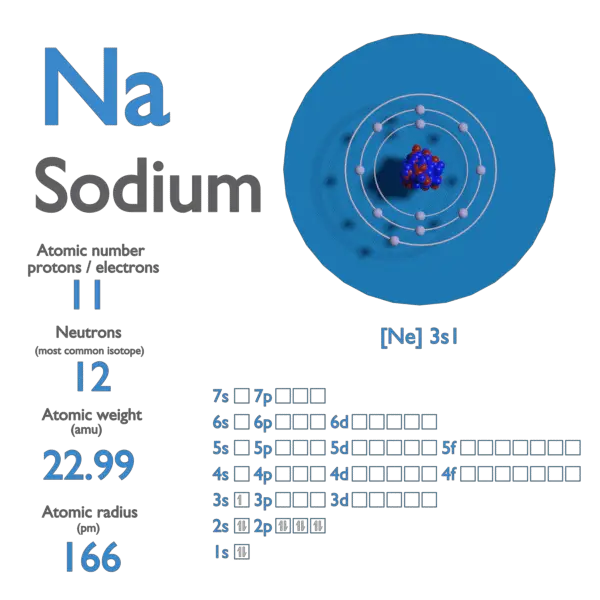
Sodium Atomic Number Atomic Mass Density Of Sodium Nuclear
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Sodium.png

Periodic Table Sodium Protons Neutrons Electrons 2023 Periodic Table
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/05/sodium-protons-neutrons-electrons-electron-configuration.png
The element Sodium has an atomic number of 11 and an average atomic mass of 22 98 which makes the mass number 23 An atomic number of 11 means this atom will have 11 protons A Nov 21 2020 nbsp 0183 32 Mass numbers of typical isotopes of Sodium are 23 The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N Neutron number plus atomic number equals
Sodium is the 11th element in the periodic table and has a symbol of Na and atomic number of 11 It has an atomic weight of 22 98977 and a mass number of 23 Sodium has eleven protons and May 28 2019 nbsp 0183 32 Given that mass number 23 and atomic number 11 Number of protons Atomic number 11 IMPORTANT POINTS Sodium is a chemical element with Atomic number 11 and Symbol Na Mass number Number of
More picture related to Mass Of Sodium Is 23 So How Many Protons And Neutrons Present In The Atom

Sodium Periodic Table Symbol
https://i.pinimg.com/originals/cf/fc/58/cffc585c3d05bf0729ecb501f068e7c7.png
Understanding Protons Electrons And Neutrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Sodium Atom Protons Neutrons Electrons
https://d1avenlh0i1xmr.cloudfront.net/a3108d07-47d4-4404-a645-de46563d93c2/17.-sodium-teachoo-01.png
Aug 11 2023 nbsp 0183 32 The isotope with mass number 23 has 23 11 or 12 neutrons because the mass number of an isotope is defined as the sum of the numbers of protons and neutrons in the For sodium that means there are 11 protons and 11 electrons Since we know that there are 11 protons there must be 12 neutrons for the mass number to equal 23 23 11 12 Answered by
Simply put with mass number 23 how many neutrons is in sodium This means that the nucleus of sodium atoms contains 11 protons Because the atom has a mass that is equal to the May 18 2021 nbsp 0183 32 How many protons neutrons and electrons does an atom of sodium 23 have An atomic number of 11 means this atom will have 11 protons A mass number of 23 means 23

Proton Neutron Electron Chart
https://physfox.s3.eu-west-2.amazonaws.com/!electricity/pne/table-pne.png
Electron Proton Neutron
https://static.vecteezy.com/system/resources/previews/014/299/902/original/atom-protons-neutrons-and-electrons-atomic-structure-consists-of-protons-neutrons-and-electrons-orbiting-the-nucleus-free-vector.jpg
Mass Of Sodium Is 23 So How Many Protons And Neutrons Present In The Atom - If we re considering 23Na atomic number 11 the stable isotope the answer to your question would be 11 protons atomic number and 12 neutrons sodium s mass is about 23 23 11 12