Lewis Structure Of Atoms Worksheet A Lewis structure is a way to show how atoms share electrons when they form a molecule Lewis structures show all of the valence electrons in an atom or molecule The valence electrons are the electrons in the outermost shell For representative elements the number of valence electrons equals the group number on the periodic table
LEWIS STRUCTURES PRACTICE WORKSHEET Draw the Lewis Structures for each of the following molecules If you are not sure if your structure is correct do a formal charge check You should consult the Lewis structure rules and a periodic table while doing this exercise A oxygens makes all atoms have 0 for FC So octet expands to 16 on the Xe Lewis Dot Structures Practice Sheet page 3 Steps for Drawing Lewis Dot Structures for Larger Molecules 1 First determine the central atom a Hydrogens H and halogens F Cl Br I are almost always outer atoms They only want to form one bond to get to a noble gas con guration
Lewis Structure Of Atoms Worksheet

Lewis Structure Of Atoms Worksheet
https://i2.wp.com/www.worksheeto.com/postpic/2010/12/lewis-dot-structure-practice-worksheet_427095.png

Atomic Structure Diagram Worksheet Atomic Structure Diagrams Atomic
https://s-media-cache-ak0.pinimg.com/originals/a0/92/b6/a092b61423485f482ee4a5ed0872efe0.jpg
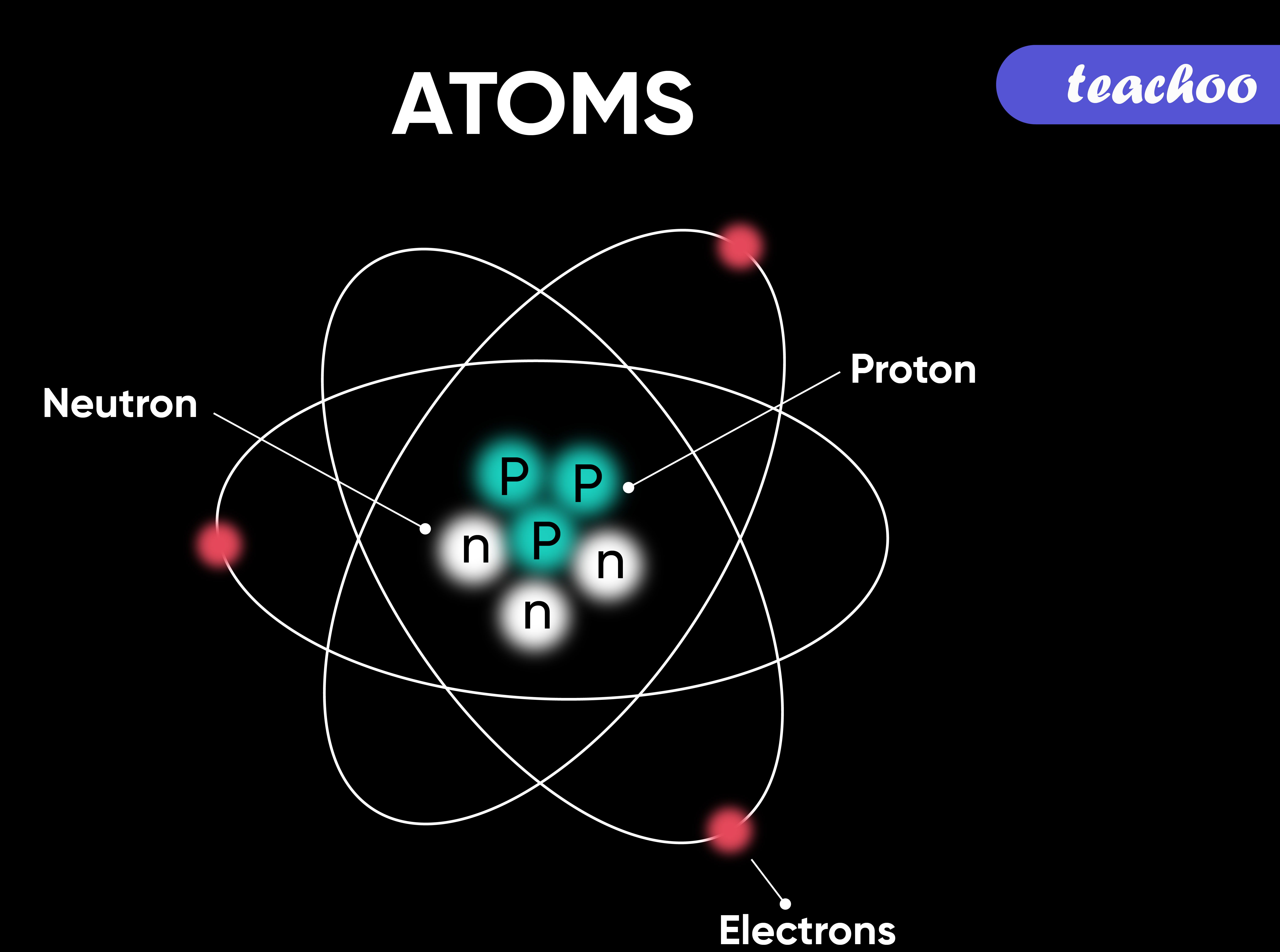
What Is Atom How Does It Exist And It s Symbols Teachoo
https://d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/30ffbef8-c92e-485a-830f-8059bcdb9ef1/atoms-teachoo.jpg
Elements in the third row of the periodic table often behave similarly to elements in the second row Use the idea of valence to construct Lewis structures of the following compounds a PH 3 b CS 2 c SiH 4 d PCl 3 Sometimes there will be more than one correct way to draw a Lewis structure for a given set of atoms The Lewis structure of a molecule is 2 dimensionl representation of a molecule It is flat and shows the relative placement of atoms and electrons connections and attachments like lone pair electrons The Lewis structure is the first step to visualizing what a molecule looks like We will follow the basic flow chart below to determine electron
The more electronegative atoms are typically farther away from the center and attaching hydrogen last is advised 4 Lone electrons not lone pairs indicate an ability to form more covalent bonds resulting in either double or triple bonds Their presence or the lack of a full octet on any atom indicates there is a better Lewis structure 5 A Lewis Structure shows the sequence in which the atoms are connected and the distribution of valence electrons around the atoms of a molecule or polyatomic ion However the Lewis Structure of a molecule or polyatomic ion does not by itself depict the three dimensional arrangement the geometry of the atoms in a molecule
More picture related to Lewis Structure Of Atoms Worksheet
20 Lewis Structures Of Atoms Worksheet Answer Key Worksheets Decoomo
https://i2.wp.com/lh5.googleusercontent.com/proxy/YOi1XmvuZwOKsI2IYqkkltxLbaSGJ0C-mWdU87l1heB5EHbp0WoiGOmubebSG6YnaUlnHTKNXu-nRREJfk8jUC3VRuiMLFNa7awTwWYwhMT5VBRU_N35YofdKD3EVpLp=w1200-h630-p-k-no-nu

Atomic Structure
http://www2.victoriacollege.edu/dept/bio/CoonsWebPages/Inorganic_chem_2020/figure_02_01_labeled.jpg
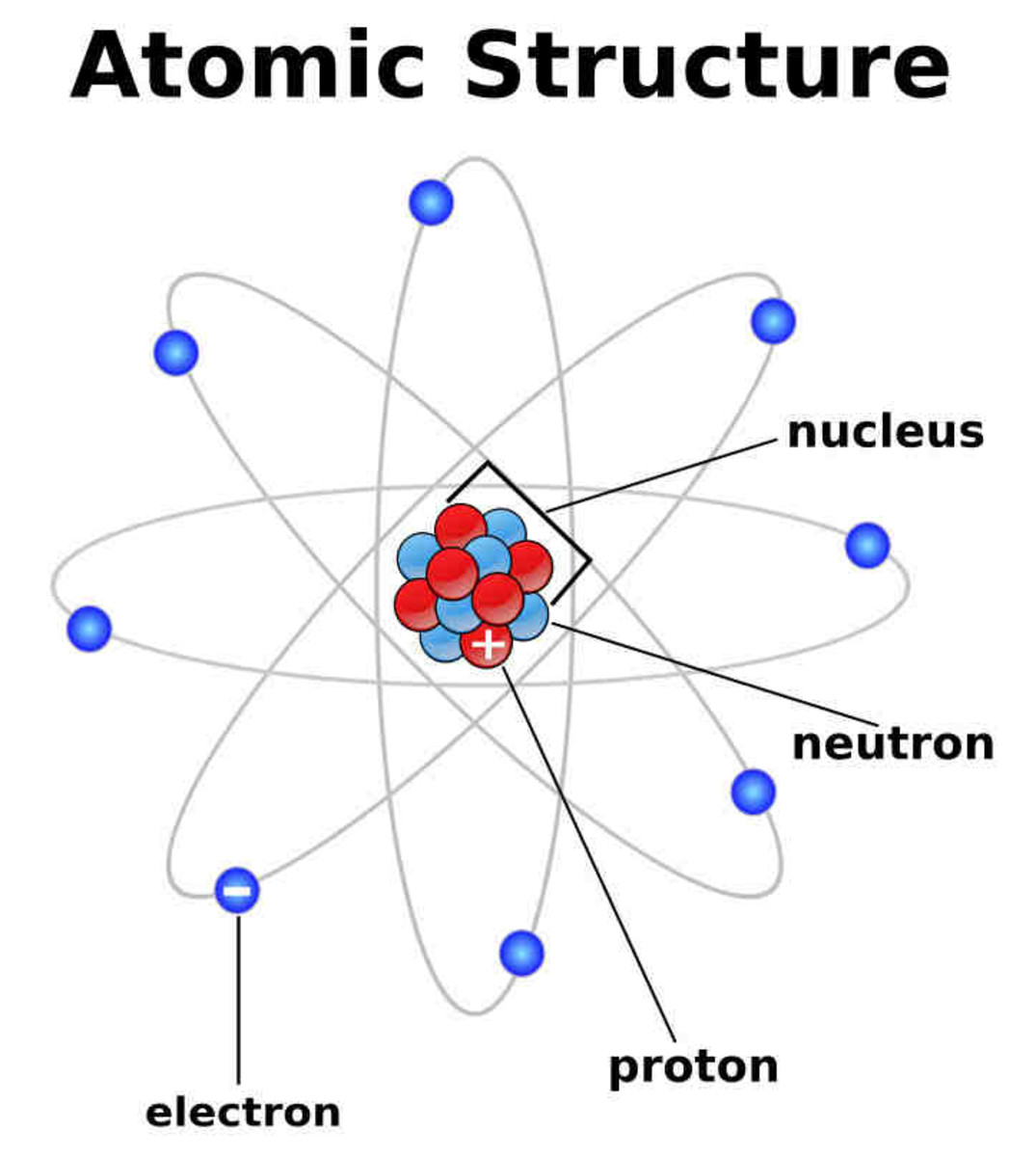
Atoms And Atomic Structure HubPages
https://usercontent1.hubstatic.com/12005272_f1024.jpg
Let us determine the Lewis structures of SiH 4 CHO 2 NO and OF 2 as examples in following this procedure Determine the total number of valence outer shell electrons in the molecule or ion For a molecule we add the number of valence electrons on each atom in the molecule SiH 4 Si 4 valence electrons atom 1 atom 4 H 1 The Lewis Structure shows how the atoms are connected to each other but does not represent bond length angles or 3D shape In a Lewis structure H will have two electrons duet rule and most other atoms will have 8 electrons noble gas configuration the octet rule but be cautious that atoms in the third period and lower can sometimes
Bonding and Lewis Structures 5 Writing Lewis Structures 1 Count the total number of valence electrons in the molecule Remember to add one for each negative charge and deduct one for each positive charge 2 Using the concept of a central atom bonded to two or more terminal atoms draw a skeleton structure joining the atoms by single bonds 3 Here are the steps to draw a Lewis structure The example is for the nitrate ion A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons The diagram is also called a Lewis dot diagram Lewis dot formula or electron dot diagram
Atomic Structure Worksheet Answer Key Structure Of Atoms Worksheet
https://lh3.googleusercontent.com/proxy/ybx5HW9_eWcBEt5KU7V5rZi5zKOvYG7hilevLaxGEmUccQJGTNQ6AWRNQ-NL7ypMDlVkO7JxC7d3-HeiAnpj9MJ9MabsvfhG9Ho=s0-d
Valence Electrons And Lewis Dot Structure Worksheet Answers Worksheet
https://imgv2-1-f.scribdassets.com/img/document/360479464/original/76f49666cd/1516979920?v=1
Lewis Structure Of Atoms Worksheet - Elements in the third row of the periodic table often behave similarly to elements in the second row Use the idea of valence to construct Lewis structures of the following compounds a PH 3 b CS 2 c SiH 4 d PCl 3 Sometimes there will be more than one correct way to draw a Lewis structure for a given set of atoms
