Kinetic Molecular Theory Worksheet Answers The word kinetic comes from a Greek word that means to move The kinetic molecular theory is based upon the assumption that atoms are in constant Random Motion State the names of the phases in order of Temperature Kinetic Energy density and bond strength between atoms Lowest Temperatures In between Temperatures Highest Temperatures
Microsoft Word Kinetic Molecular Theory Worksheet Key docx Kinetic Molecular Theory Worksheet 1 The word kinetic comes from a Greek word that means to move The kinetic molecular theory is based upon the assumption that particles of matter atoms or molecules are in constant random motion Explain your answer Solution Describe what happens to the average kinetic energy of ideal gas molecules when the conditions are changed as follows a The pressure of the gas is increased by reducing the volume at constant temperature b The pressure of the gas is increased by increasing the temperature at constant volume
Kinetic Molecular Theory Worksheet Answers

Kinetic Molecular Theory Worksheet Answers
http://hannahtuition.com/wp-content/uploads/2013/04/Lower-Sec-Kinetic-Particle-theory-and-Atoms_Page_1-724x1024.jpg

Kinetic Molecular Theory Worksheets
https://i2.wp.com/smithfieldjustice.com/wp-content/uploads/2020/02/kinetic-molecular-theory-worksheet-7.jpg

50 Kinetic Molecular Theory Worksheet Chessmuseum Template Library
https://i.pinimg.com/originals/d7/a9/24/d7a9247b824bfecc0a257784ef1cd9bb.jpg
The Kinetic Molecular Theory Explains the Behavior of Gases Part I Recalling that gas pressure is exerted by rapidly moving gas molecules and depends directly on the number of molecules hitting a unit area of the wall per unit of time we see that the KMT conceptually explains the behavior of a gas as follows Amontons s law Describe kinetic molecular theory 3 Identify each statement as True or False According to the basic assumption of kinetic molecular theory gas particles a are far apart b have a significant volume with respect to the volume of the container they occupy c move rapidly in a constant random motion
Use kinetic molecular theory to explain the difference between evaporation and boiling of a liquid Q6 Use the chart to answer the following questions This page titled Kinetic Theory of Gases Worksheet is shared under a CC BY NC SA 4 0 license and was authored Q8 Freon 12 is used as a refrigerant in central home air conditioners The rate of effusion of Freon 12 to Freon 11 molar mass 137 4 g mol is 1 07 1 Which formula is correct for Freon 12 CF4 C F 4 CF3Cl C F 3 C l CF2Cl2 C F 2 C l 2 CFCl3 C F C l 3 or CCl4 C C l 4 Hint use Graham s Law of Effusion
More picture related to Kinetic Molecular Theory Worksheet Answers
Solved Name Date Class Gases And Kinetic Molecular Theory Chegg
https://media.cheggcdn.com/study/4b3/4b36dadd-ed52-44b6-90c2-331d4506dc14/image

SOLUTION Chemistry Class Notes Kinetic Molecular Theory 1 Studypool
https://sp-uploads.s3.amazonaws.com/uploads/services/2008336/20220119120215_61e7fdc702712_chemistry_class_notes__kinetic_molecular_theory_1_page0.png

PPT Kinetic Molecular Theory PowerPoint Presentation Free Download
https://image3.slideserve.com/6901460/kinetic-molecular-theory-n.jpg
Kinetic Molecular Theory Supplemental Worksheet 1 What assumptions do we make when using the ideal gas laws Are the assumptions always true 2 Consider 2 gases A and B each in a 1 0 L container with both gases at the same temperature and pressure The mass of gas A in the container is 0 25 g and the mass of gas B in the container is 0 A real gas can be approximated by an ideal gas at low density low pressure high temperature An Ideal Gas is a theoretical model of a gas It is defined by the following assumptions The molecules are point particles with negligible volume The molecules obey the laws of mechanics An ideal gas can not be liquefied or solidified
Chemistry library 17 units 48 skills Unit 1 Atoms compounds and ions Unit 2 Mass spectrometry Unit 3 Chemical reactions and stoichiometry Unit 4 More about chemical reactions Unit 5 Electronic structure of atoms Unit 6 Periodic table Unit 7 Chemical bonds Unit 8 Gases and kinetic molecular theory Theory an accepted statement about science that has been tested and peer reviewed many times that explains observations facts can be used to make predictions Kinetic this word means movement Kinetic energy is energy that has to do with things moving and is stored in objects To examine means to look at or study

Top 10 Kinetic Theory Worksheet Templates Free To Download In PDF Format
https://data.formsbank.com/pdf_docs_html/309/3091/309140/page_1_thumb_big.png
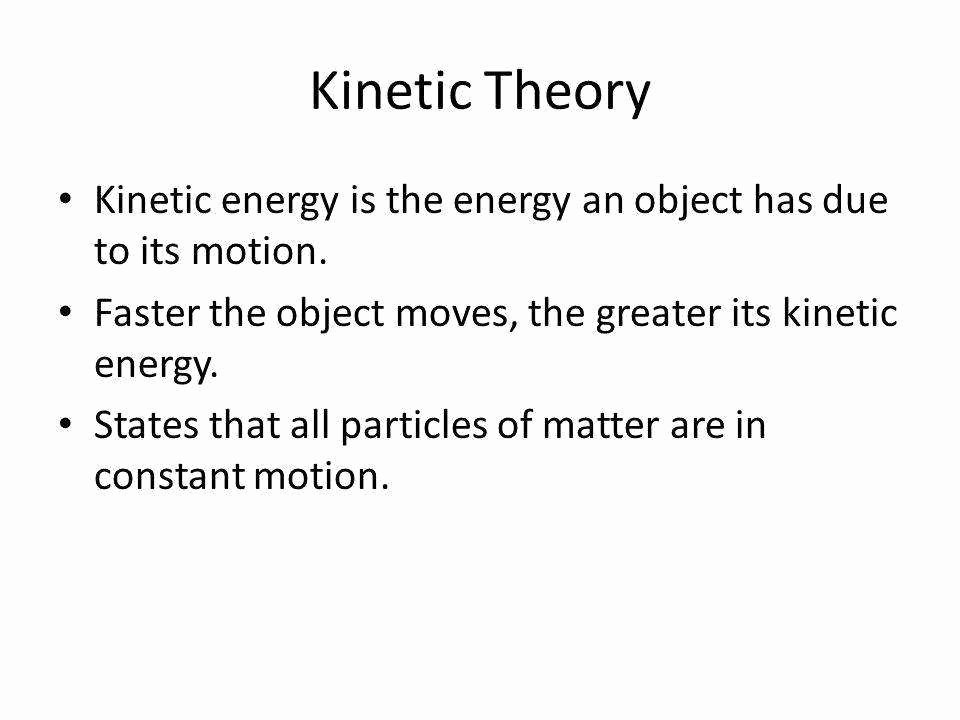
50 Kinetic Molecular Theory Worksheet
https://chessmuseum.org/wp-content/uploads/2019/10/kinetic-molecular-theory-worksheet-fresh-kinetic-molecular-theory-worksheet-of-kinetic-molecular-theory-worksheet.jpg
Kinetic Molecular Theory Worksheet Answers - Describe kinetic molecular theory 3 Identify each statement as True or False According to the basic assumption of kinetic molecular theory gas particles a are far apart b have a significant volume with respect to the volume of the container they occupy c move rapidly in a constant random motion
