Isotope With 3 Protons And 5 Neutrons 3 days ago nbsp 0183 32 An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties
Sep 13 2019 nbsp 0183 32 Get the definition of an isotope See examples of isotopes and learn the difference between an isotope and a nuclide of an element Feb 4 2020 nbsp 0183 32 Every element on the periodic table has multiple isotope forms The chemical properties of isotopes of a single element tend to be nearly identical the exceptions are the isotopes of hydrogen since the number of neutrons has such a significant effect on the size of the hydrogen nucleus
Isotope With 3 Protons And 5 Neutrons

Isotope With 3 Protons And 5 Neutrons
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Fluorine.png

Lithium Protons Neutrons Electrons Electron Configuration
https://material-properties.org/wp-content/uploads/2020/09/Lithium-protons-neutrons-electrons-configuration.png

Beryllium Atomic Number Atomic Mass Density Of Beryllium
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Beryllium.png
Aug 19 2022 nbsp 0183 32 For example the naturally occurring isotope carbon 14 present in water is used to understand the age of water and other organic materials Read the IAEA Bulletins to learn more about the importance and uses of isotopes and their signatures An isotope is a variation of an element that possesses the same atomic number but a different mass number A group of isotopes of any element will always have the same number of protons and electrons
An isotope is named after the element and the mass number of its atoms For example carbon 12 is an isotope of carbon with a mass number of 12 All three isotopes of hydrogen have identical Jan 2 2013 nbsp 0183 32 According to the definition of isotope each of the same element has the same atomic number Z but each has a different mass number A The atomic number corresponds to the number of protons in the atomic nucleus of the atom
More picture related to Isotope With 3 Protons And 5 Neutrons

Isotope Notation Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/isotope_notation_calculations.jpeg

An Ion Has 19 Protons 20 Neutrons And 18 Electrons What Is The
https://media.brainly.com/image/rs:fill/w:750/q:75/plain/https://us-static.z-dn.net/files/d4c/ca54ba48dd747cd7db9650b14e3b313d.png

Uranium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/09/Uranium-protons-neutrons-electrons-configuration.png
In this concept tutorial learn about what an isotope is some common isotopes and their uses and how isotopes form and breakdown Summary Isotopes are atoms that have the same atomic number but different mass numbers due to a change in the number of neutrons The term nuclide refers to the nucleus of a given isotope of an element The atomic mass of an atom equals the sum of the protons and the neutrons
[desc-10] [desc-11]

Chemistry Review Coloring Pages Editable Chemistry Worksheets
https://i.pinimg.com/originals/39/16/0f/39160fdc69574123f4a644bdadbc91d7.jpg
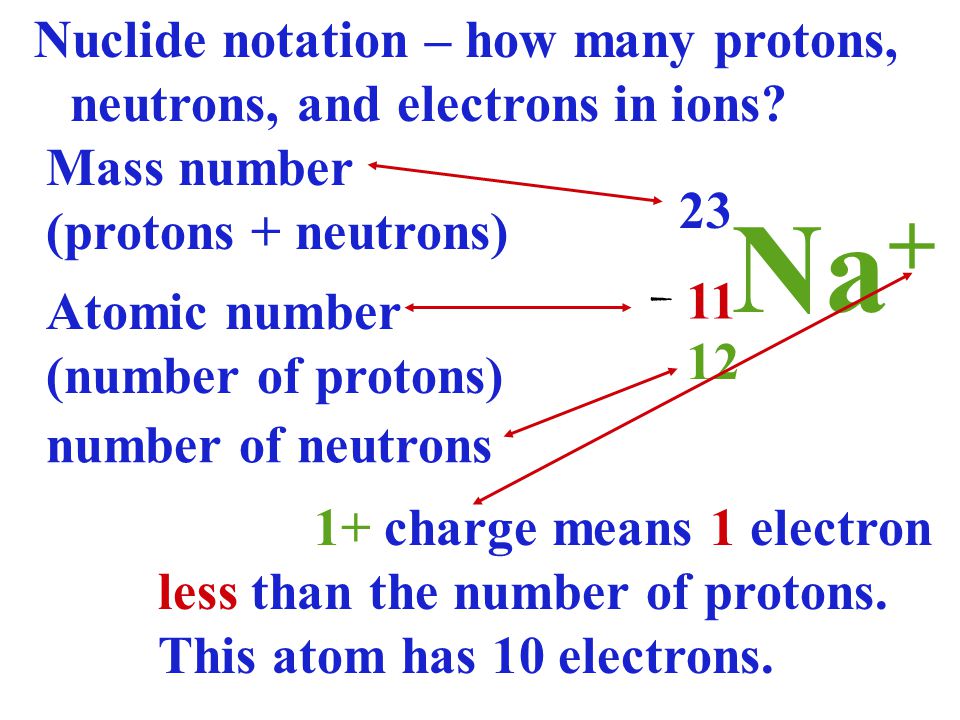
SimplyChemistry C1 1 2 PROTON NUMBER MASS NUMBER IONS ISOTOPES
https://3.bp.blogspot.com/-xjR7quI6CFs/VYLYdrkjBiI/AAAAAAAABeA/I1ufwOUUHhA/s1600/slide_9.jpg
Isotope With 3 Protons And 5 Neutrons - [desc-14]