How To Do Combined Gas Law Apr 5 2021 nbsp 0183 32 The combined gas law is an ideal gas law that combines Boyle s law Charles s law and Gay Lussac s law It states the the ratio between the pressure volume product and absolute temperature of a gas is a constant
Sep 28 2024 nbsp 0183 32 The combined gas law combines the three gas laws Boyle s Law Charles Law and Gay Lussac s Law It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant Jul 12 2023 nbsp 0183 32 To use the ideal gas law to describe the behavior of a gas In this module the relationship between Pressure Temperature Volume and Amount of a gas are described and how these relationships can be combined to give a general expression that describes the behavior of
How To Do Combined Gas Law
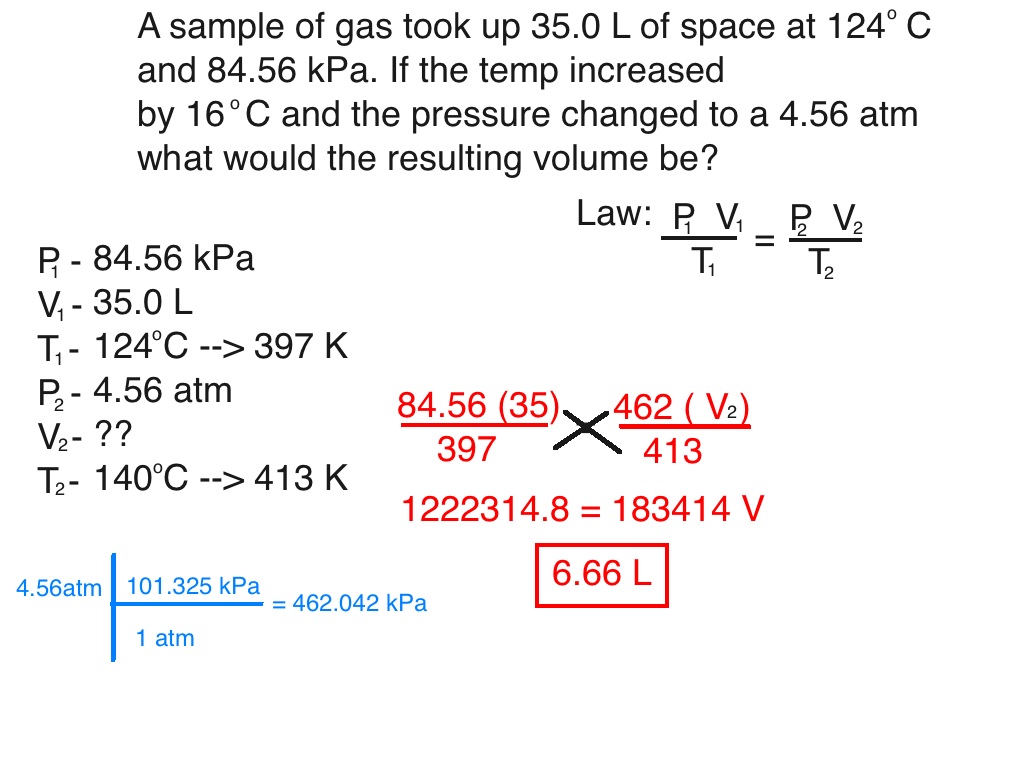
How To Do Combined Gas Law
http://chemistry101efhs.weebly.com/uploads/2/9/9/7/29971125/893174_orig.jpg
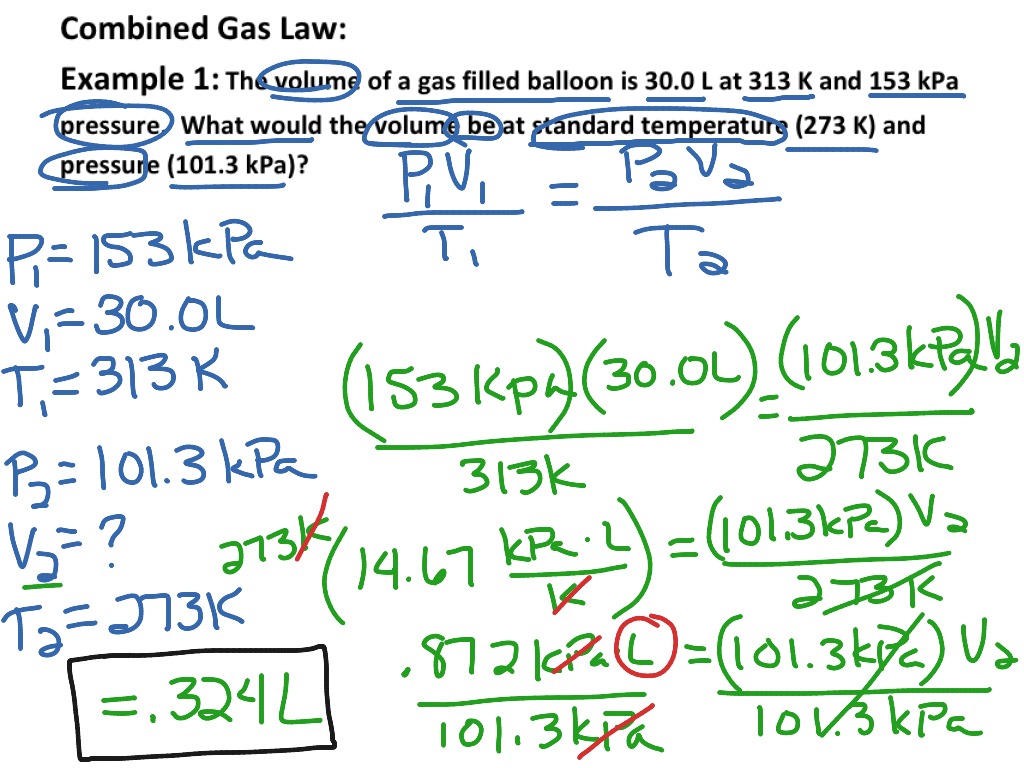
ShowMe Derivation Of Combined Gas Law
https://showme0-9071.kxcdn.com/files/10398/pictures/thumbs/906596/last_thumb1367444863.jpg
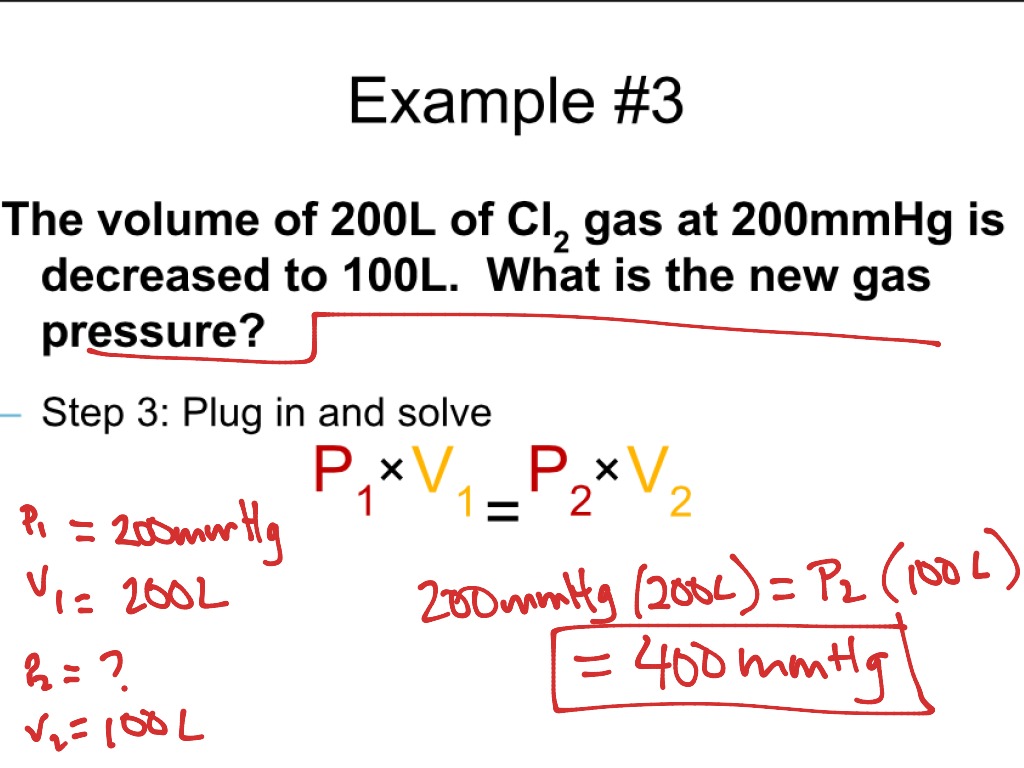
ShowMe Derivation Of Combined Gas Law
https://showme0-9071.kxcdn.com/files/601610/pictures/thumbs/1543952/last_thumb1399578699.jpg
Using the Combined Gas Law There are two ways to use the combined gas law The easiest way is to derive the Boyle s Charles or the Gay Lussac law If the temperature is kept constant the combined gas law reverts to P1V1 P2V2 That s Boyle s law When we fix the pressure we have V1T1 V2T2 which is Charles law Combined Gas Law Formula Combined gas law can be mathematically expressed as k PV T Where P pressure T temperature in kelvin V volume K constant units of energy divided by temperature When two substances are compared in two different conditions the law can be stated as P i V i T i P f V f T f Where P i initial
The combined gas law relates pressure temperature and volume when everything else is held constant mainly the moles of gas n The most common form of the equation for the combined gas law is as follows Here is one way to quot derive quot the Combined Gas Law Step 1 Write the problem solving form of Boyle s Law Step 2 Multiply by the problem solving form of Charles Law Step 3 Multiply by the problem solving form of Gay Lussac s Law Step 4 Take
More picture related to How To Do Combined Gas Law
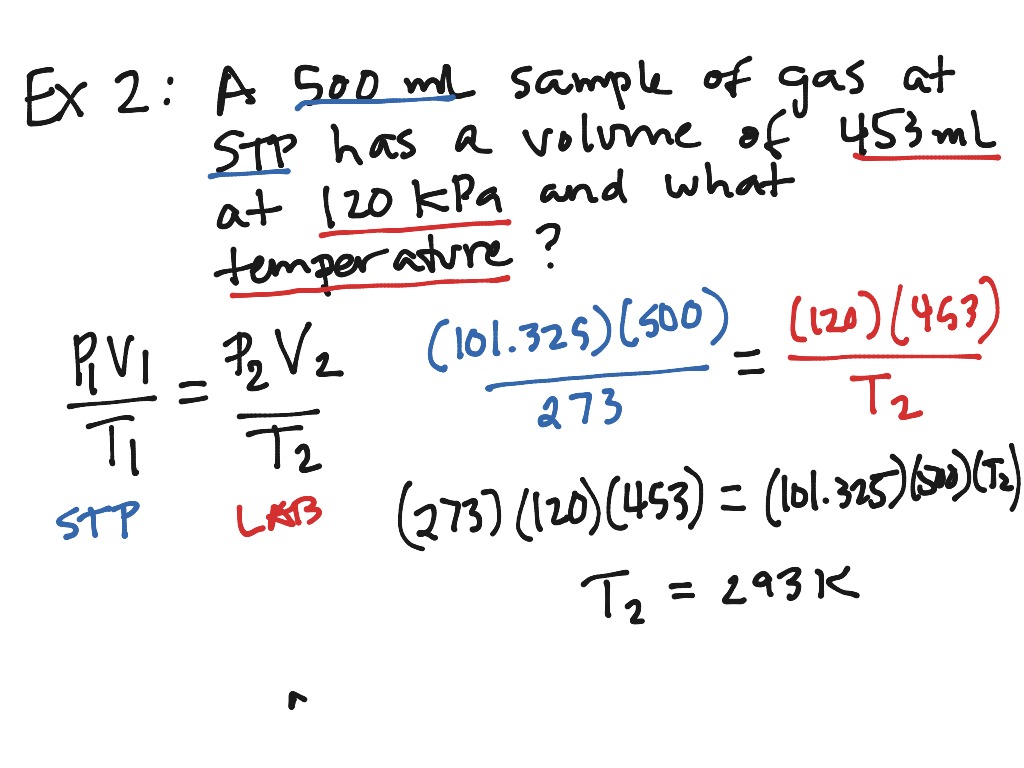
Ideal Gas Law Equation Example
https://asiasupergrid.com/images/ideal-gas-law-equation-example.jpg
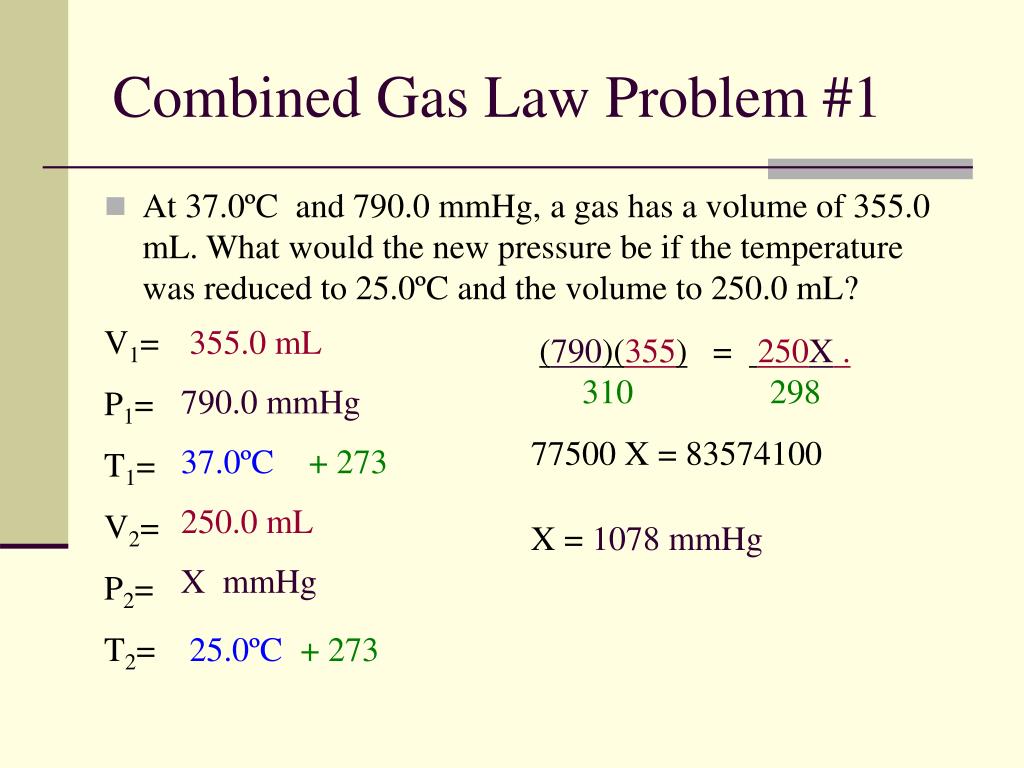
PPT Properties Of Gases Gas Laws PowerPoint Presentation Free
https://image3.slideserve.com/5712074/slide27-l.jpg
Gas Laws Presentation Chemistry
http://www.sliderbase.com/images/referats/89b/(7).PNG
Use the combined gas law to determine the relationships between pressure volume and temperature of a gas One thing we notice about all gas laws collectively is that volume and pressure are always in the numerator and temperature is always in the denominator Therefore the formula of combined gas law is PV T K Where P pressure T temperature V volume K is constant One can adjust the formula for the combined gas law so as to compare two sets of conditions in one substance
Sep 17 2017 nbsp 0183 32 This chemistry video tutorial explains how to solve combined gas law problems This video contains many examples with all of the formulas and equations that Feb 11 2024 nbsp 0183 32 The Combined Gas Law is a useful equation for relating initial and final conditions of a gas sample Visit https www Breslyn for more Gas Laws help a

Solving Combined Gas Law Problems Charles Law Boyle s Law Lussac s
http://i.ytimg.com/vi/CgrCFRsWMtI/maxresdefault.jpg
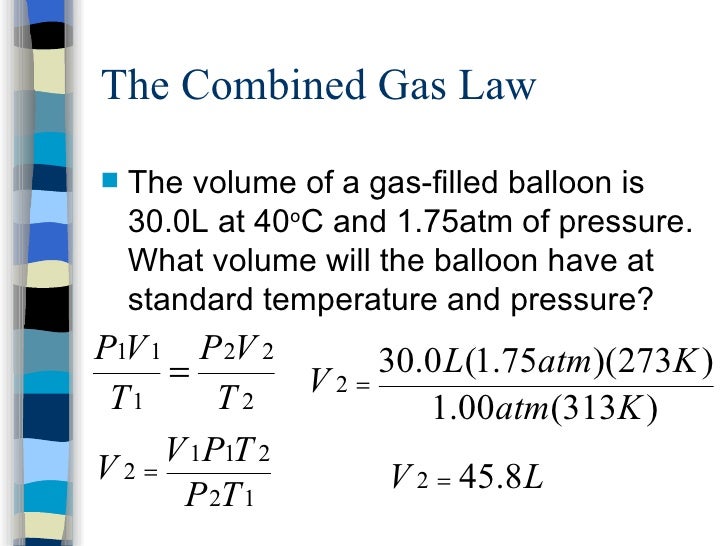
Notes Gas Laws
https://image.slidesharecdn.com/notesgaslaws-101012103038-phpapp01/95/notes-gas-laws-20-728.jpg?cb=1286880012
How To Do Combined Gas Law - The combined gas law relates pressure temperature and volume when everything else is held constant mainly the moles of gas n The most common form of the equation for the combined gas law is as follows
.PNG)