How To Calculate The Relative Atomic Mass Of An Element From Isotopic Abundances Oct 22 2024 nbsp 0183 32 Learn how to calculate relative atomic mass for IGCSE Chemistry using isotope mass numbers and abundance with worked examples
How do we know what the percent abundance for each of the isotopes of a given element Isotopes are separated through mass spectrometry MS traces show the relative abundance of isotopes vs mass number mass charge ratio To determine relative atomic mass we simply multiply each isotopic mass by its abundance add all the values together and divide the total value by 100 percent Effectively we are calculating
How To Calculate The Relative Atomic Mass Of An Element From Isotopic Abundances

How To Calculate The Relative Atomic Mass Of An Element From Isotopic Abundances
https://i.ytimg.com/vi/ID4RSzgg0Nk/maxresdefault.jpg

Calculating Percent Abundance And Average Atomic Mass YouTube
https://i.ytimg.com/vi/WkQWVyohYxA/maxresdefault.jpg
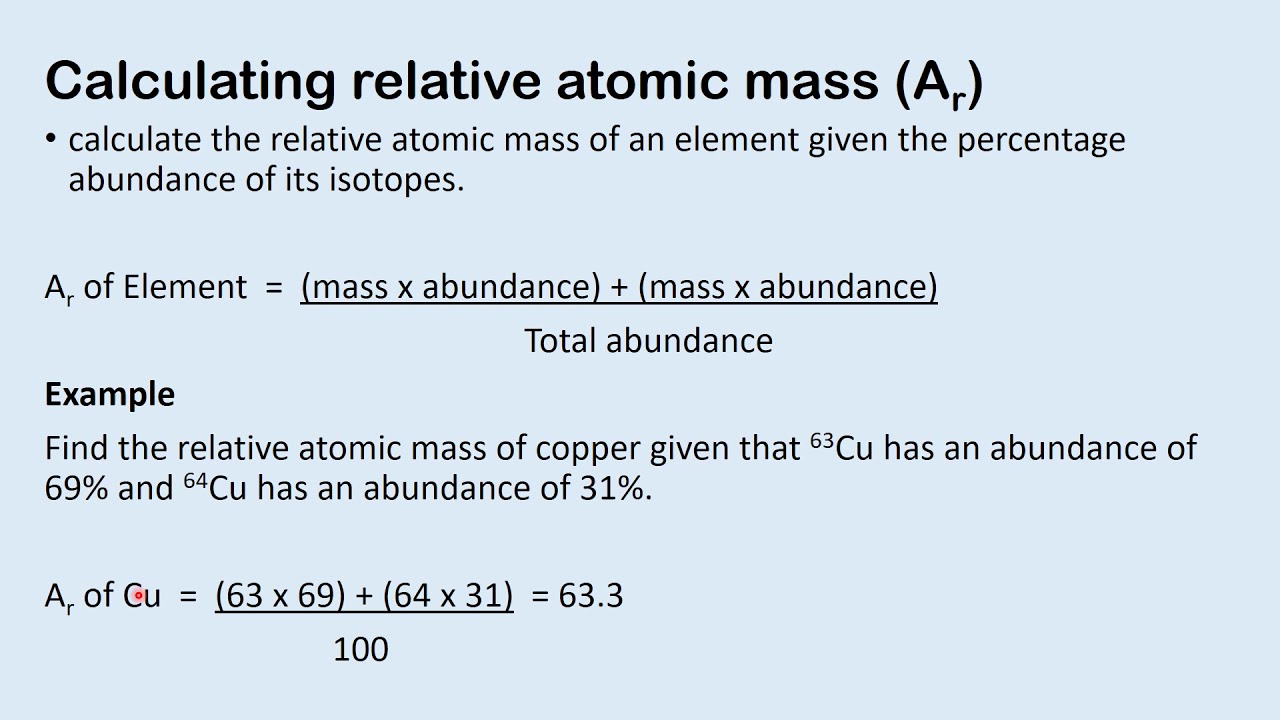
Calculating Relative Atomic Mass YouTube
https://i.ytimg.com/vi/_BwoVpijvlU/maxresdefault.jpg
It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element It also looks at the problems thrown up by elements with How do you determine and calculate isotope abundance when you know the relative atomic mass also known as atomic weight as measured in amu or atomic mass numbers Here we will go through the algebra and reasoning to
Oct 25 2013 nbsp 0183 32 Many a times students taking GCE A Level H2 Chemistry are required to calculate the Relative Atomic Mass of an element with given information on Isotopic Abundance The abundance of an isotope is the Dec 12 2024 nbsp 0183 32 Relative atomic mass A r is calculated using a formula that accounts for the isotopic masses and their natural abundances This method reflects the weighted average of isotopes present in a sample
More picture related to How To Calculate The Relative Atomic Mass Of An Element From Isotopic Abundances

S1 2 2 Calculating Relative Atomic Mass YouTube
https://i.ytimg.com/vi/DjJqgx0HzyY/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYACzgWKAgwIABABGH8gEygUMA8=&rs=AOn4CLDnmI1WSowCC55lOda-IAl5ExzMdA

How To Calculate Mass Of An Isotope From Isotopic Mass And Percentage
https://i.ytimg.com/vi/N9Lp6RFk5pg/maxresdefault.jpg

Calculate Relative Abundance And Mass Number Of Unknown Isotope Under
https://i.ytimg.com/vi/eGL_6YkNHkQ/maxresdefault.jpg
Jan 8 2025 nbsp 0183 32 The relative atomic mass of isotopes is calculated by determining the weighted average of the masses of isotopes taking into account their relative abundances According to Feb 7 2025 nbsp 0183 32 Use the following formula for relative abundance chemistry problems M1 x M2 1 x M E M1 is the mass of one isotope x is the relative abundance M2 is the mass of the second isotope and M E is the
May 25 2022 nbsp 0183 32 e g calculate the relative atomic mass for chlorine given that it has 2 isotopes 35 Cl with an abundance of 75 and 37 Cl with an abundance of 25 A r 35 x 75 37 x 25 100 35 5 We can also use the mass To calculate the atomic mass of an isotope we need to know its isotopic abundance as well as the masses of its individual isotopes Each isotope s contribution to the total atomic mass

Easy Trick To Learn Atomic Mass 1 To 30 Elements YouTube
https://i.ytimg.com/vi/kkqLl0UEjs8/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGHIgQigwMA8=&rs=AOn4CLAFLIzA26YYz2TERxuxlu1xTnWcMA
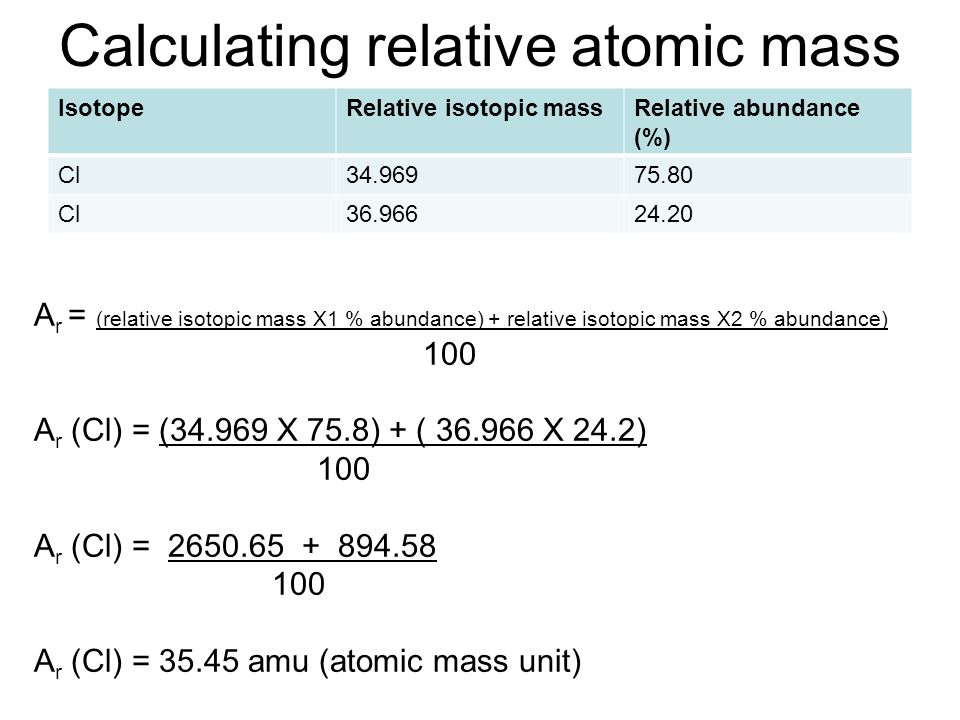
Calculating relative atomic mass Dynamic Periodic Table Of Elements
https://periodictable.me/wp-content/uploads/2018/08/Calculatingrelativeatomicmass.jpg
How To Calculate The Relative Atomic Mass Of An Element From Isotopic Abundances - The relative atomic mass is worked out using the following formula illustrated for two isotopes where the abundances are given in percentage values A r frac mass 1