How Set Timer On Apple Watch This docket will identify specifically what types of documents or parts of documents are acceptable for submission in electronic form without paper records and the agency receiving unit s e g specific center office division branch to which such submissions may be made
May 7 2025 nbsp 0183 32 In this guide we will break down what 21 CFR Part 11 actually requires what companies need to put in place to stay compliant and how a modern Quality Management System QMS can support ongoing compliance without adding unnecessary complexity 21 CFR Part 11 Compliance Checklist is a set of regulatory guidance to companies on creating and managing digital records and signatures from document forms manuals to workflows 21 CFR Part 11 Compliance Checklist is mandated by Food and Drug Administration in
How Set Timer On Apple Watch
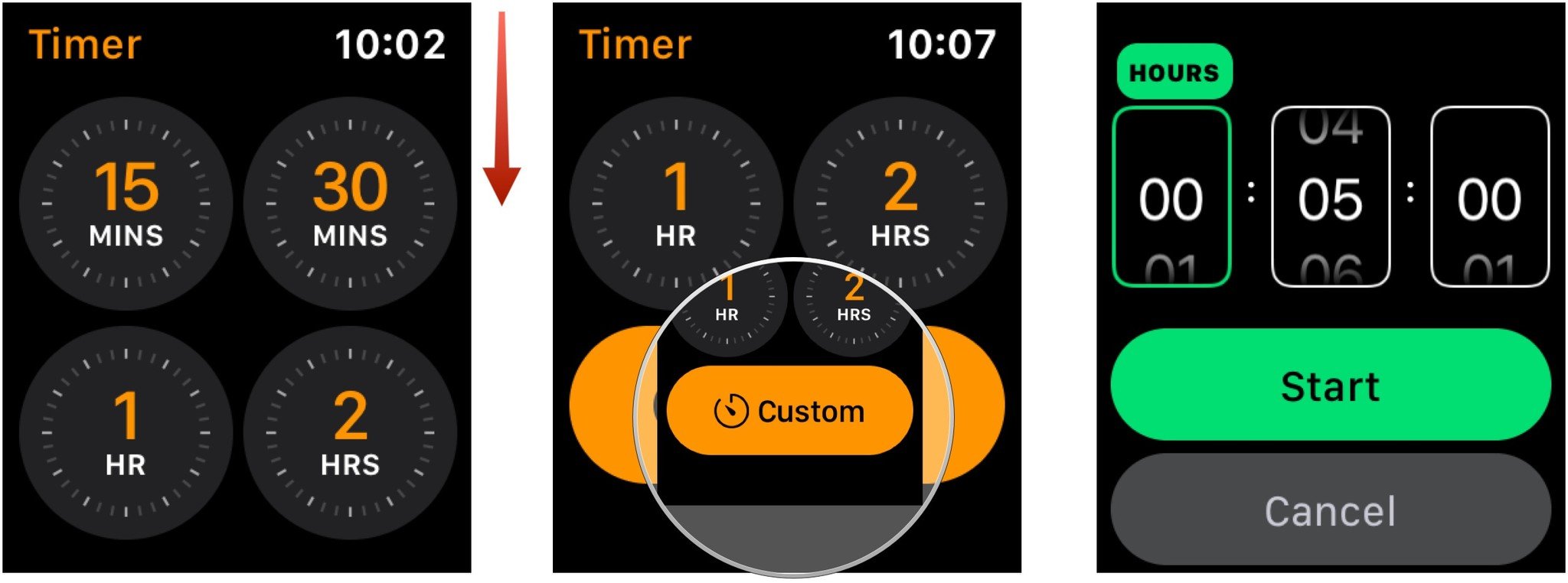
How Set Timer On Apple Watch
https://www.imore.com/sites/imore.com/files/styles/large/public/field/image/2019/07/apple-watch-timer-custom.jpg?itok=r68ThAMd

So Stellen Sie Einen Timer Auf Der Apple Watch Ein Tech News
https://static1.xdaimages.com/wordpress/wp-content/uploads/wm/2023/04/apple-watch-set-timer-feature.jpg

Apple Watch Ultra Supports Standard Apple Watch Bands
https://cdn.mobilesyrup.com/wp-content/uploads/2022/09/apple-watch-ultra-header-resized-2-20220908-scaled.jpg
Aug 6 2025 nbsp 0183 32 Preparing the 24 documents FDA requires for software and cybersecurity can be daunting Here is a list and quick guide on best practices Compliance with 21 CFR Part 11 is key To do this certain documents are vital for data integrity and security in regulated industries Let s look at some of these documents What Documents Need To Be 21 CFR Part 11 Compliant First there s the Electronic Records Policy
Below is a list of major changes that the FDA implemented The documentation level determines what documentation the FDA requires in a product submission and what activities need to occur during the development process Oct 24 2024 nbsp 0183 32 Learn everything you need to know about 21 CFR Part 11 compliance including electronic signatures electronic records and implementation requirements for FDA regulated industries
More picture related to How Set Timer On Apple Watch
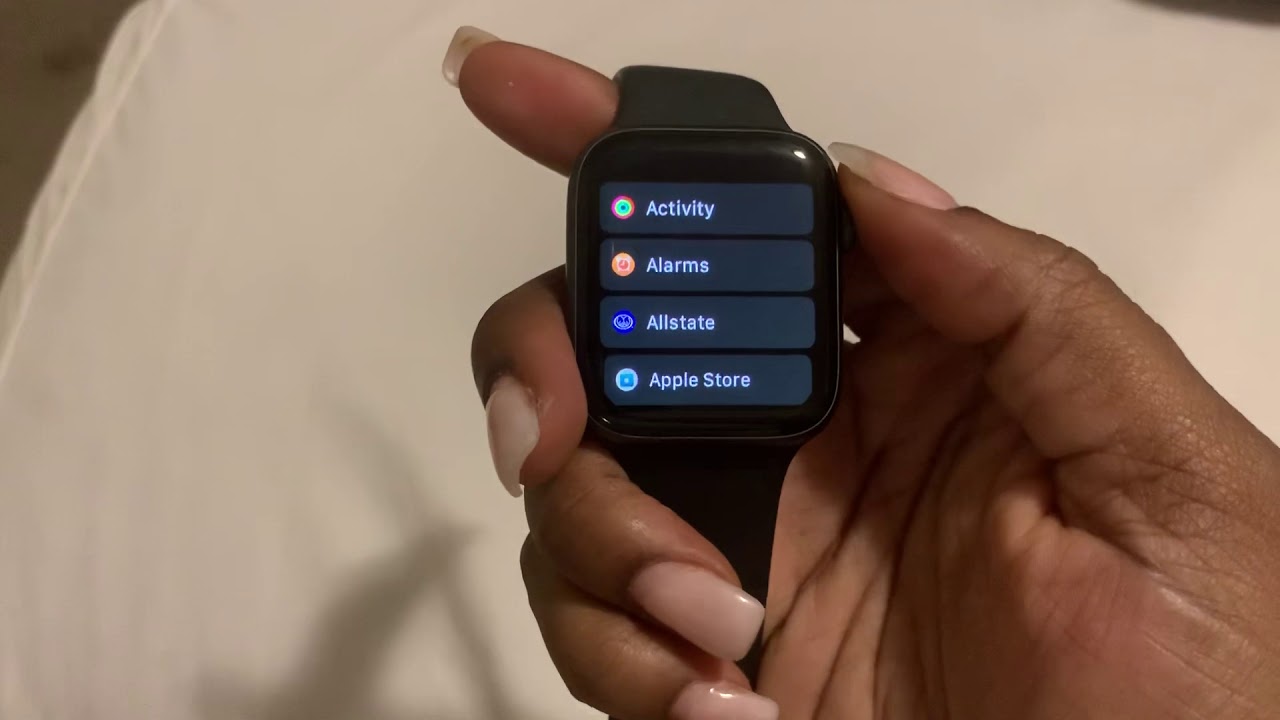
How To Set An Alarm Or Timer On Apple Watch YouTube
https://i.ytimg.com/vi/xof_8W43iTc/maxresdefault.jpg

Apple Watch Countdown And Timer Figma
https://s3-alpha.figma.com/hub/file/1734737581/da696e3a-eb9e-4691-8e37-99fa38d66166-cover.png

How To Set A Timer For The Apple Watch YouTube
https://i.ytimg.com/vi/J2A1L2CpEmc/maxresdefault.jpg
Write useful and effective documents that provide direction and evidence of compliance and quality Explore vital FDA 21 CFR Part 11 compliance data integrity security and streamlined solutions through Tricentis Vera for secure electronic records
[desc-10] [desc-11]

How To Set Timer On Apple Watch Simple Steps
https://www.beepinghand.com/wp-content/uploads/2023/08/How-to-Set-Timer-on-Apple-Watch-1024x555.jpg

How To Set A Custom Timer On Apple Watch LaptrinhX
https://www.howtogeek.com/wp-content/uploads/2021/04/Setting-a-Timer-on-Apple-Watch.png?height=200p&trim=2,2,2,2
How Set Timer On Apple Watch - [desc-14]