How Law Of Conservation Of Mass Works Dec 9 2023 nbsp 0183 32 The Law of Conservation of Mass is a fundamental concept in chemistry stating that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations According to the law the mass of the reactants in a chemical reaction equals the mass of the products
The law of conservation of mass states that mass within a closed system remains the same over time Discover more about the law of conservation of mass including its importance equations and some examples of this law in action Sep 20 2022 nbsp 0183 32 The law of conservation of mass states that during a chemical reaction the total of the products must be equal to the total of the reactants Describe an example of the law of conservation of mass
How Law Of Conservation Of Mass Works

How Law Of Conservation Of Mass Works
https://i.pinimg.com/originals/c8/44/be/c844be7c94078b3e32dcbaf6f9fead43.png

The Law Of Conservation Of Mass Kidpid
https://www.kidpid.com/wp-content/uploads/2018/08/The-law-of-conservation-of-mass.jpg

Newsela The Conservation Of Matter During Physical And Chemical Changes
https://media.newsela.com/article_media/extra/NewselaConservationMass.jpg?width=750&compression=85
In chemistry the law of conservation of mass states that the mass of the products the chemical substances created by a chemical reaction will always equal the mass of the reactants the substances that make the chemical reaction Think of it Conservation of mass principle that the mass of an object or collection of objects never changes no matter how the constituent parts rearrange themselves Mass has been viewed in physics in two compatible ways
The total mass of chemicals before and after a reaction remains the same This is called the Law of Conservation of Mass Jun 3 2024 nbsp 0183 32 According to the law of conservation of mass mass is neither created nor destroyed during a chemical reaction For example when coal is burned the carbon atom in it changes into carbon dioxide The carbon atom changes from a solid to a gas yet its mass remains constant
More picture related to How Law Of Conservation Of Mass Works
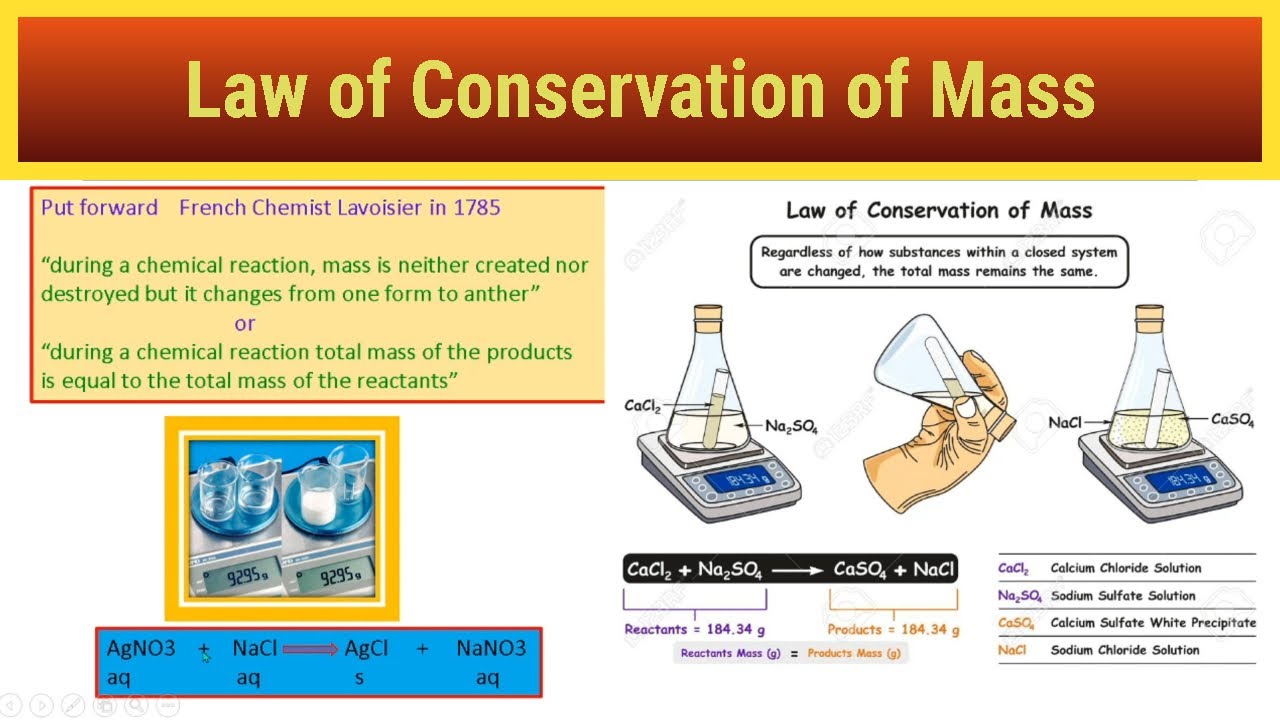
LAW OF CONSERVATION OF MASS EXPLAINED BY NI Concepts YouTube
https://i.ytimg.com/vi/qqk04MeZsTU/maxresdefault.jpg

The Law Of Conservation Of Mass Stock Image A500 0829 Science
https://media.sciencephoto.com/image/a5000829/800wm/A5000829-The_Law_of_Conservation_of_Mass.jpg
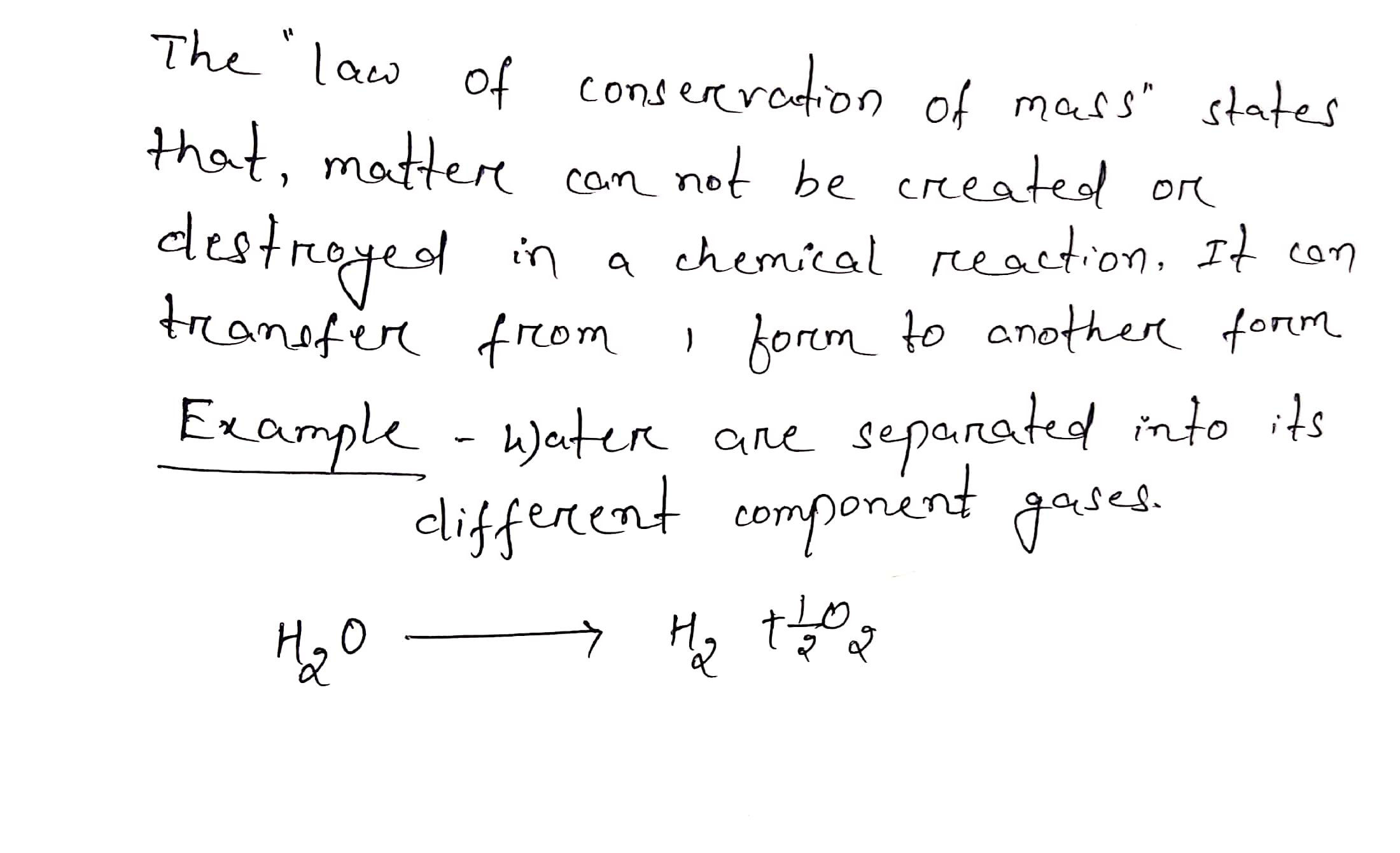
Explain Law Of Conservation Of Mass With Example
https://d1hj4to4g9ba46.cloudfront.net/questions/1397640_1232743_ans_ec949ee12848458dbce180d31eae20ee.jpg
The law of conservation of mass finds its particle foundation in the fact that chemical reactions involve the rearrangement of atoms and the conservation of atoms Because atoms are conserved so is mass Jan 21 2025 nbsp 0183 32 The law of conservation of mass is also known as the quot law of indestructibility of matter quot Example PageIndex 1 If heating 10 grams of ce CaCO3 produces 4 4 g of ce CO2 and 5 6 g of ce CaO show that these observations are in agreement with the law of conservation of mass
The law of conservation of mass states that in a chemical reaction between any object the mass that is produced by them is neither created nor destroyed The carbon atom changes from a complex solid structure to a simple gas but yet its mass remains the same Dec 21 2020 nbsp 0183 32 The law of conservation of mass states that in a closed system including the whole universe mass can neither be created nor destroyed by chemical or physical changes In other words total mass is always conserved
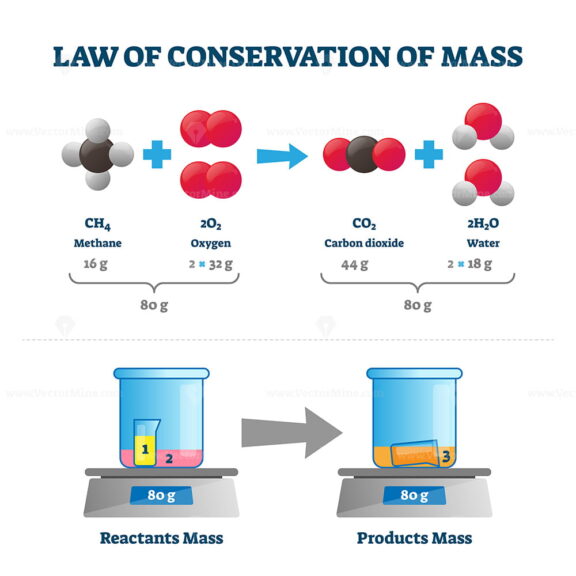
Law Of Conservation Of Mass Vector Illustration VectorMine
https://vectormine.b-cdn.net/wp-content/uploads/Law_of_Conservation_of_Mass-576x576.jpg
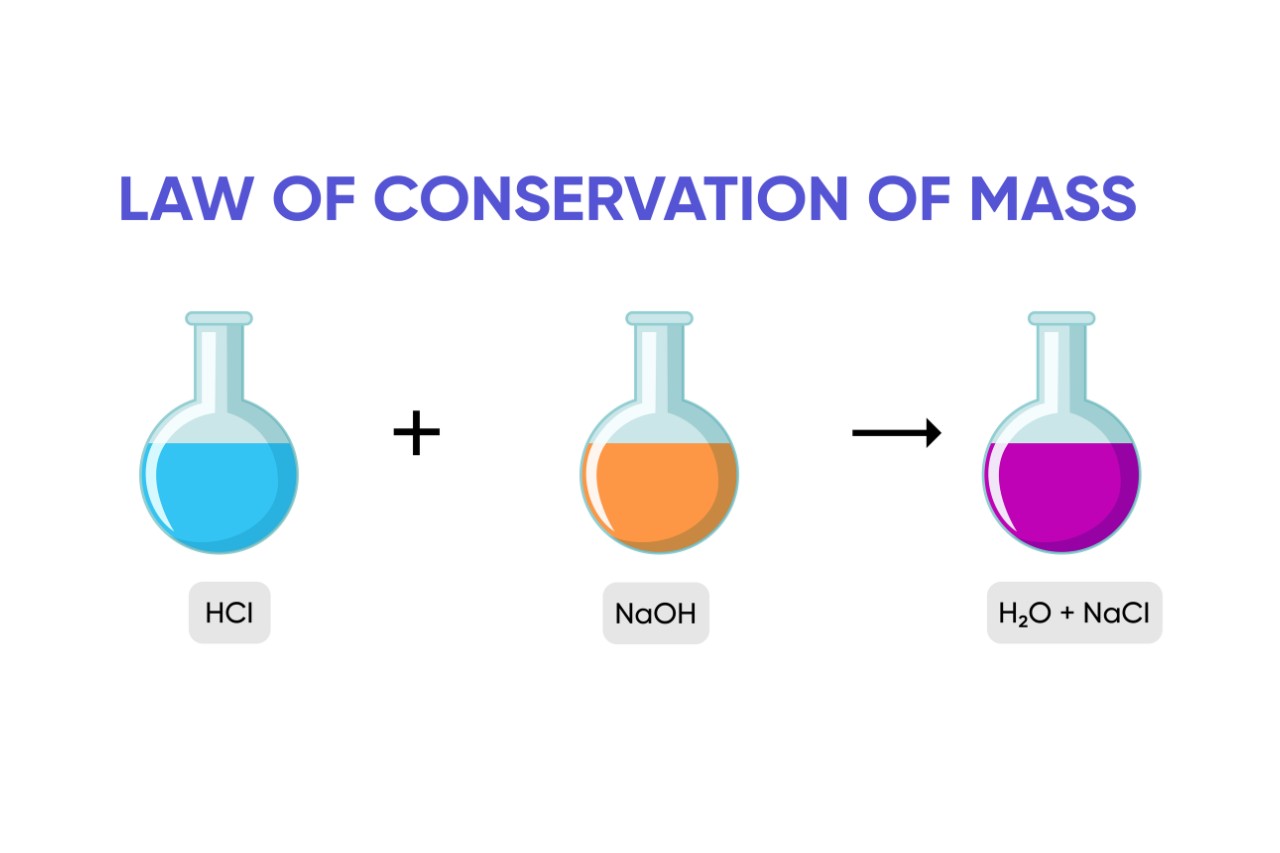
13 Captivating Facts About Law Of Conservation Of Mass Facts
https://facts.net/wp-content/uploads/2023/09/13-captivating-facts-about-law-of-conservation-of-mass-1694346453.jpg
How Law Of Conservation Of Mass Works - The Law of Conservation of Mass or Matter in a chemical reaction can be stated thus In a chemical reaction matter is neither created nor destroyed It was discovered by Antoine Laurent Lavoisier 1743 94 about 1785