How Does Temperature Affect Collision Theory Jan 10 2021 nbsp 0183 32 Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates The Arrhenius equation describes the relation between a reaction s rate constant and its activation energy temperature and dependence on collision orientation
Jul 3 2015 nbsp 0183 32 At a higher temperature the particles have a higher kinetic energy making their collisions more frequent therefore increasing the chances of a successful collision This speeds up the reaction rate as well This page describes and explains the way that changing the temperature affects the rate of a reaction It assumes that you are already familiar with basic ideas about the collision theory and with the Maxwell Boltzmann distribution of molecular energies in a gas
How Does Temperature Affect Collision Theory
How Does Temperature Affect Collision Theory
https://image2.slideserve.com/3873529/collision-theory-n.jpg
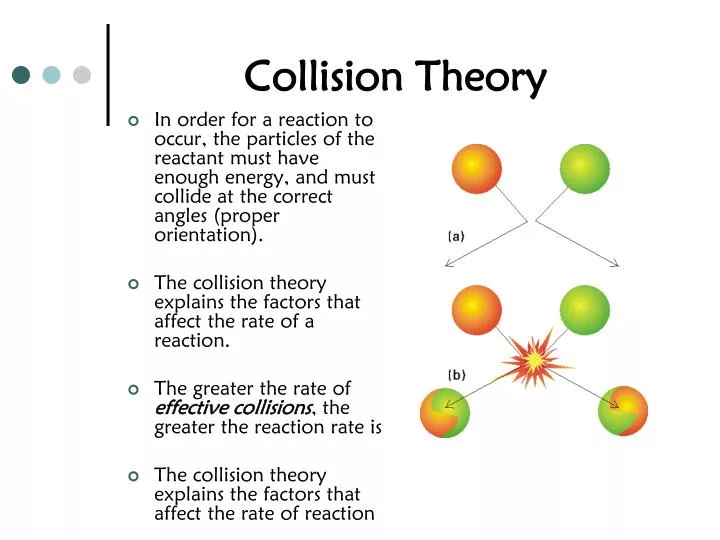
Question Video Recalling How Parameters In Collision Theory Affect
https://media.nagwa.com/546193845092/en/thumbnail_l.jpeg

Collision Theory Concentration Temperature And Surface Area GCSE
https://i.ytimg.com/vi/HjlxUBWvxH4/maxresdefault.jpg
Sep 29 2024 nbsp 0183 32 The effect of temperature on collisions is not so straightforward as concentration or surface area a small increase in temperature causes a large increase in rate For aqueous and gaseous systems a rough rule of thumb is that for every 10 o C increase in temperature the rate of reaction approximately doubles Mar 15 2014 nbsp 0183 32 Temperature changes both the frequency and effectiveness of collisions Collision theory is related to the kinetic molecular theory This explains how all matter is made of particles and those particles are in constant motion
Jan 19 2024 nbsp 0183 32 The effect of temperature on collisions is not so straightforward as concentration or surface area a small increase in temperature causes a large increase in rate For aqueous and gaseous systems a rough rule of thumb is that for every 10 o C increase in temperature the rate of reaction approximately doubles If the temperature is raised the kinetic energies of both A and B are increased so that there are more collisions per second and a greater fraction of these will lead to chemical reaction The rate therefore generally increases with increasing temperature A catalyst can be thought of as an agent which alters the speed of a chemical reaction
More picture related to How Does Temperature Affect Collision Theory

Kinetics Collision Theory A Level ChemistryStudent
https://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory2.png

Kinetics Collision Theory A Level ChemistryStudent
https://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory4.png
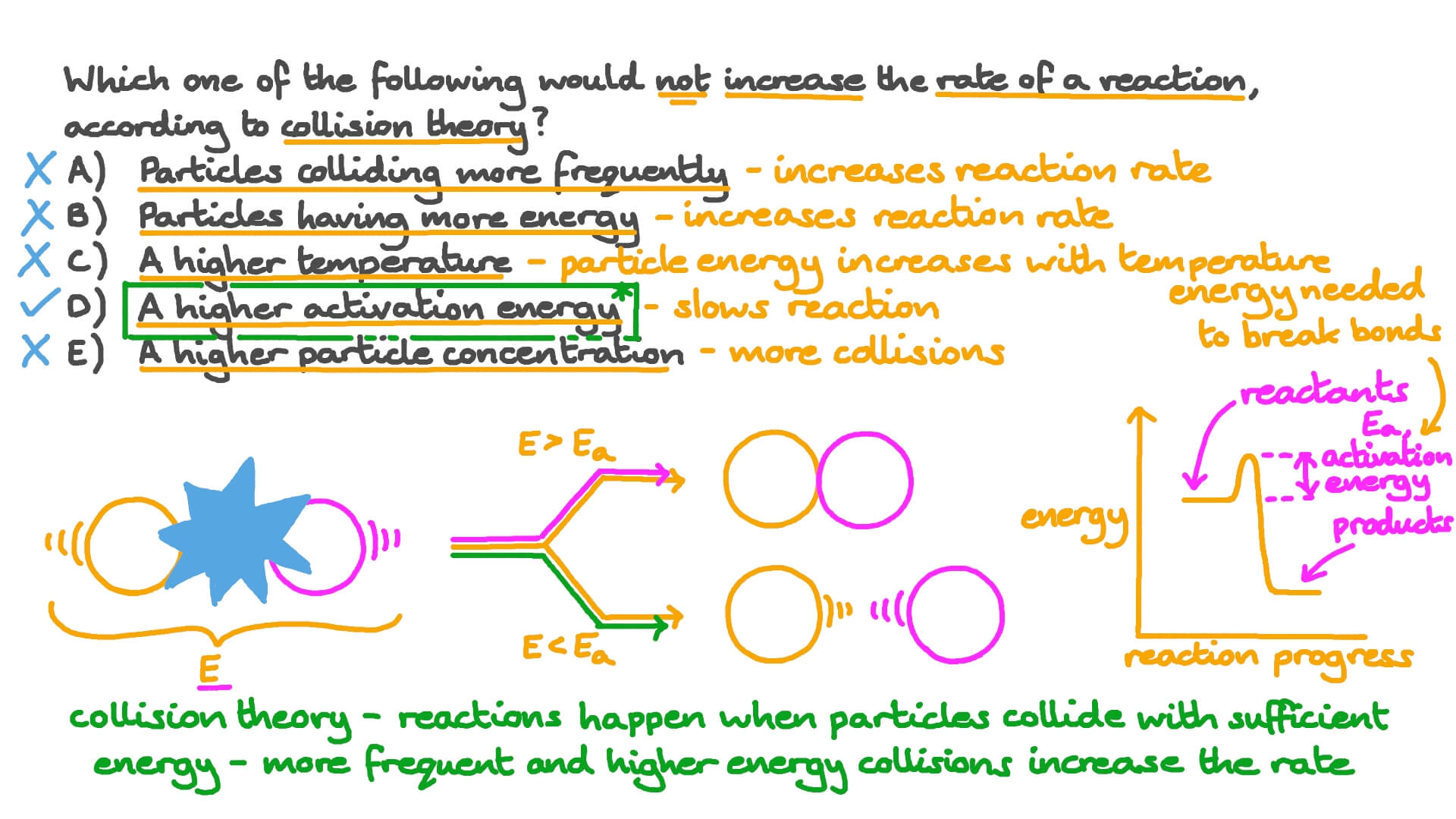
Collision Theory
https://mmerevise.co.uk/wp-content/uploads/2022/10/Collision-theory-1024x771.png
Use the postulates of collision theory to explain the effects of physical state temperature and concentration on reaction rates Use the postulates of collision theory to explain the effects of physical state temperature and concentration on reaction rates Define the concepts of activation energy and transition state Use the Arrhenius equation in calculations relating rate constants to temperature
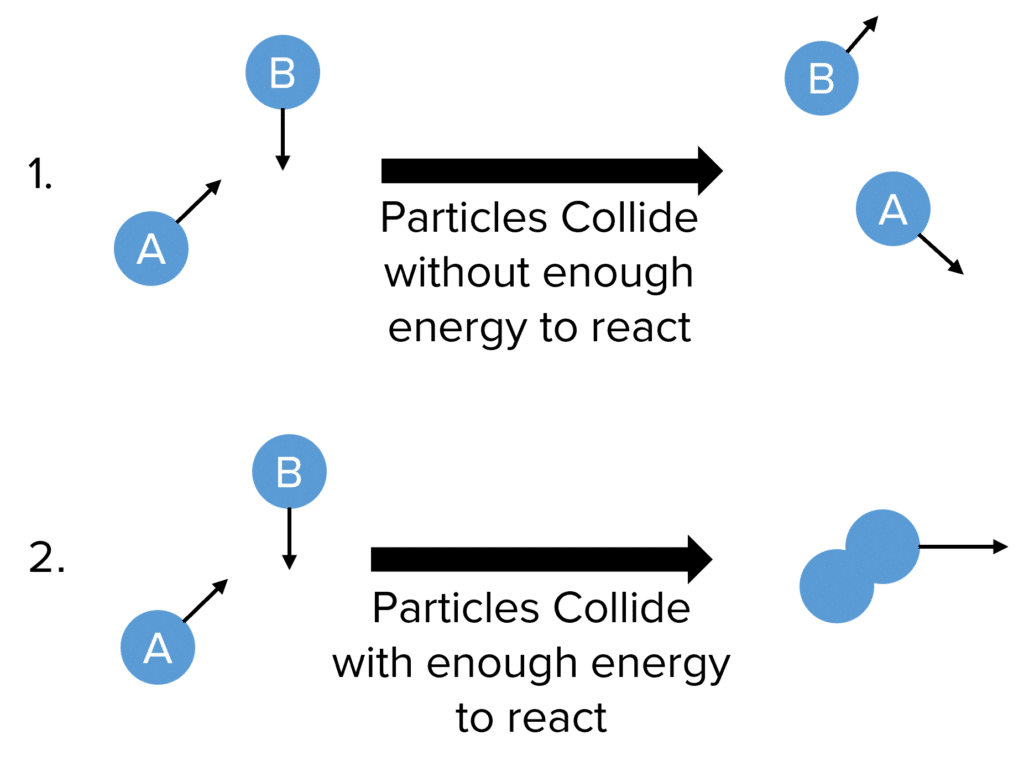
How does an increase in temperature affect the rate of reaction Explain this effect in terms of the collision theory of the reaction rate The rate of a certain reaction doubles for every 10 176 C rise in temperature Feb 18 1999 nbsp 0183 32 Kinetic theory says that molecules are in constant motion The kinetic energy and molecule velocity increase with temperature KE 1 2 mv 2 Reactions usually require collisions between reactant molecules or atoms The formation of
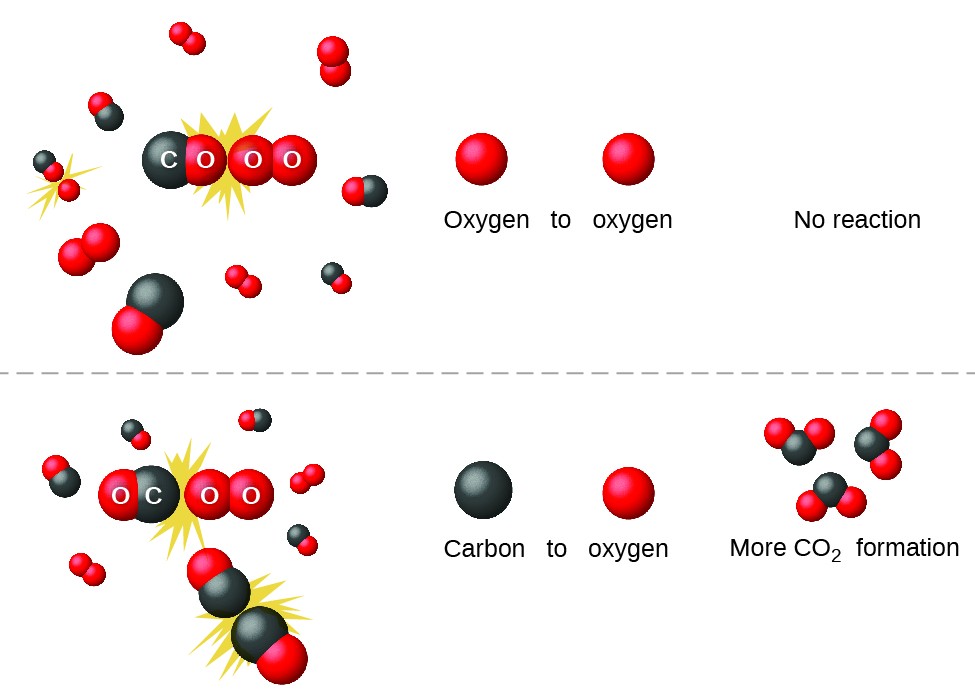
Collision Theory
https://s3-us-west-2.amazonaws.com/courses-images-archive-read-only/wp-content/uploads/sites/887/2015/04/23205758/CNX_Chem_12_05_COandO2.jpg
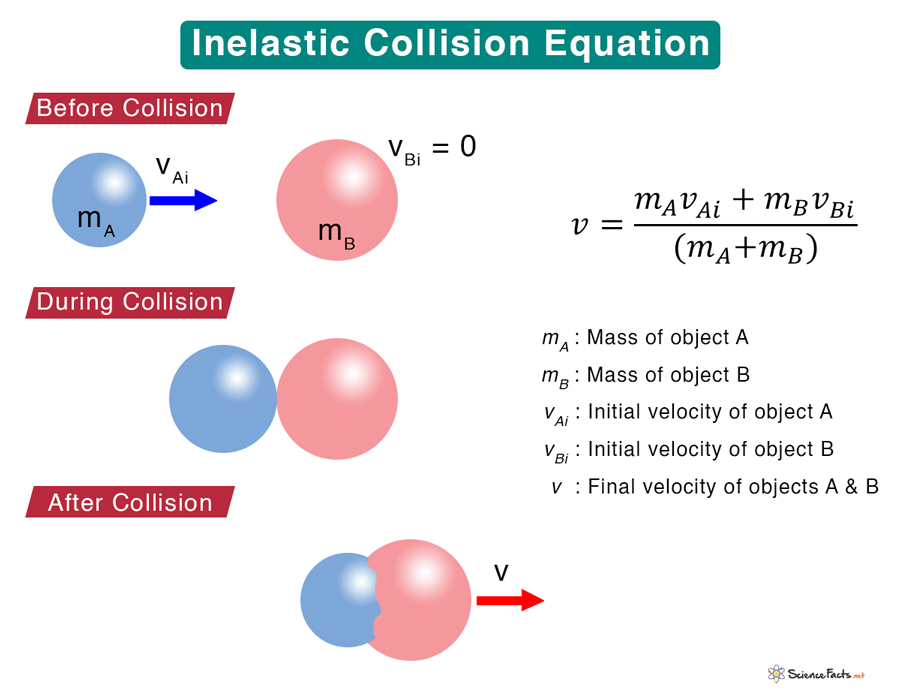
Inelastic Collision Definition Formula And Examples
https://www.sciencefacts.net/wp-content/uploads/2022/02/Inelastic-collision-formula.jpg
How Does Temperature Affect Collision Theory - Conversely lowering temperature has the opposite effect Therefore temperature influences reaction rate via two ways Firstly it affects the average speed of reactant particles and molecules which in turn affects the collision rate between them