Heat Capacity Exercises With Answers Practice problems on heat capacity and specific heat Examples of how to determine the heat heat capacity and change of temperature
Specific Heat Capacity Quiz A The thermal energy change gain or loss in a substance is equal to the amount of substance 215 shc 215 temperature change rise or fall E mc T 2 T 1 or E mc T or E mc or Q mc which are all the same equation Use the one familiar to May 28 2020 nbsp 0183 32 Homes may be heated by pumping hot water through radiators What mass of water will provide the same amount of heat when cooled from 95 0 to 35 0 176 C as the heat provided when 100 g of steam is cooled from 110 176 C to 100 176 C Answer
Heat Capacity Exercises With Answers
Heat Capacity Exercises With Answers
https://media.nagwa.com/474167959048/en/thumbnail_l.jpeg

Specific Heat Worksheet Answer Key
https://static.docsity.com/documents_first_pages/2021/04/20/8383defce09a810692f71a4f70fe05f4.png

Specific Heat Capacity Worksheet W answers Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/236e9c8b-cc06-4ea1-aa23-bad749825b8a/Coverphoto.png
Answers 1 What does it mean if water has a higher specific heat capacity than oil It requires more energy to raise its temperature by the same amount 2 What is the difference between thermal energy and temperature Temperature is a measure of the average kinetic energy of the molecules present Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24 5oC to 26 4oC How much heat did this sample absorb c for water 4 18 J goC ans 2 66 kJ 2 How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25 0oC to 10 0oC ans 14 1 kJ 3
Latent heat and Specific heat capacity questions 1 How much water at 50 176 C is needed to just melt 2 2 kg of ice at 0 176 C 2 How much water at 32 176 C is needed to just melt 1 5 kg of ice at 10 176 C 3 How much steam at 100 176 is needed to just melt 5 kg of ice at 15 176 C 4 A copper cup holds some cold water at 4 176 C Answers mc T where Q heat energy m mass and T change in temp Remember T Tfinal Tinitial Show all work and proper units A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 176 C to 175 176 C Calculate the specific heat capacity of iron
More picture related to Heat Capacity Exercises With Answers
Heat Capacity Of Calorimeter Deon has Edwards
https://i.ytimg.com/vi/nrpWz2pjO-s/maxresdefault.jpg
1 3 Specific Heat Capacity
https://s2.studylib.net/store/data/018037878_1-5dc8c711621f05e80a2a3975e8530b10.png
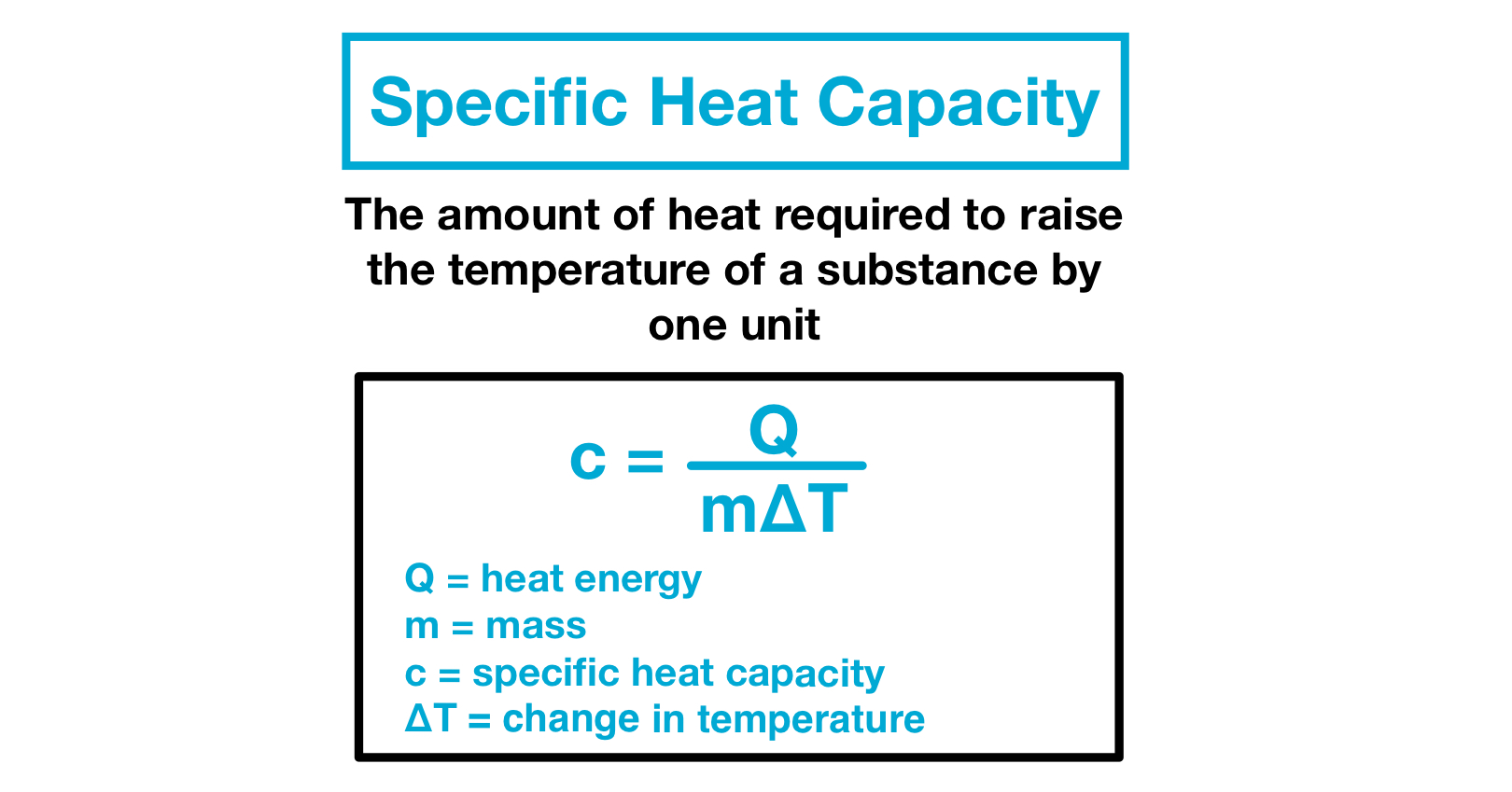
Specific Heat Capacity Definition AbramsrEwing
https://d20khd7ddkh5ls.cloudfront.net/heat_capacity.jpeg
In this set of practice questions we will go over the main types of questions on calorimetry including the heat capacity the heat of reaction finding the final temperature of a mixture constant pressure calorimetry and constant volume calorimetry Specific Heat Worksheet DIRECTIONS Use q m T Cp to solve the following problems Show all work and units 1 A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 176 C to 175 176 C Calculate the specific heat capacity of iron 2
Specific heat capacity of copper 390 J kg 1 K 1 a If the temperature of the water rises from 15 176 C to 35 176 C calculate the thermal energy gained by the water Worksheet 12 2 Latent Heat Specific Heat and Work 1 How much heat is absorbed by 100 g of ice at 10 C to become water at 20 C 2 A 200 g sample of water at 80 C is heated to steam at 120 C How much heat does it absorb 3 How much heat is needed to change 2 kg of ice at 50 C into steam at 150 C 4

Heat Calculations Worksheet With Solutions Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/677f994510877a7c683793f2196badd3.png?v=1653667687

KSSM TINGKATAN 4 HEAT SPECIFIC HEAT CAPACITY EXERCISES LATIHAN
https://i.ytimg.com/vi/awm-YKAEiJo/maxresdefault.jpg
Heat Capacity Exercises With Answers - Answers 1 What does it mean if water has a higher specific heat capacity than oil It requires more energy to raise its temperature by the same amount 2 What is the difference between thermal energy and temperature Temperature is a measure of the average kinetic energy of the molecules present