Gmp Documentation Guidelines Good Documentation Practices commonly referred to as GDPs are the guidelines that one follows in recording information in a legible traceable and reproducible manner
There are two primary types of documentation used to manage and record GMP compliance instructions directions requirements and records reports Appropriate good documentation Feb 26 2025 nbsp 0183 32 Learn all about the good documentation practice including basics GMP document preparation issuance and retrieval of records recording of time correction of entries handling
Gmp Documentation Guidelines

Gmp Documentation Guidelines
https://i.ytimg.com/vi/7MIquKWDqd4/maxresdefault.jpg
What Is GMP Good Manufacturing Practices SafetyCulture 49 OFF
https://gmp.com.vn/upload_images/images/2023/06/05/GMP_contents_02.png

GMP Good Manufacturing Practice LMG New York 60 OFF
https://www.compliancequest.com/wp-content/uploads/2023/02/10-principles-of-gmp.jpg
Apr 26 2025 nbsp 0183 32 The goal of GMP documentation is to systematically record manage and control activities that directly or indirectly influence the quality of medicinal products Oct 13 2021 nbsp 0183 32 GMP documentation guidelines are important to ensure compliance accuracy and quality in your operations and record keeping Learn the guidelines here
Apr 20 2024 nbsp 0183 32 Understanding implementing and enforcing good documentation practices guidelines is of the utmost importance for pharmaceutical manufacturers So keep reading as This chapter describes the underlying principles of proper documentation for GMP operations to assist the user while working with GMP activities These guidelines should be helpful for
More picture related to Gmp Documentation Guidelines

What Are GMP Guidelines Good Manufacturing Practices For 43 OFF
https://i.ytimg.com/vi/TcWyGapJ1TQ/maxresdefault.jpg

Good Documentation Practices GDP And GMP Definition Importance
https://i.ytimg.com/vi/Ht5APL23sEw/maxresdefault.jpg
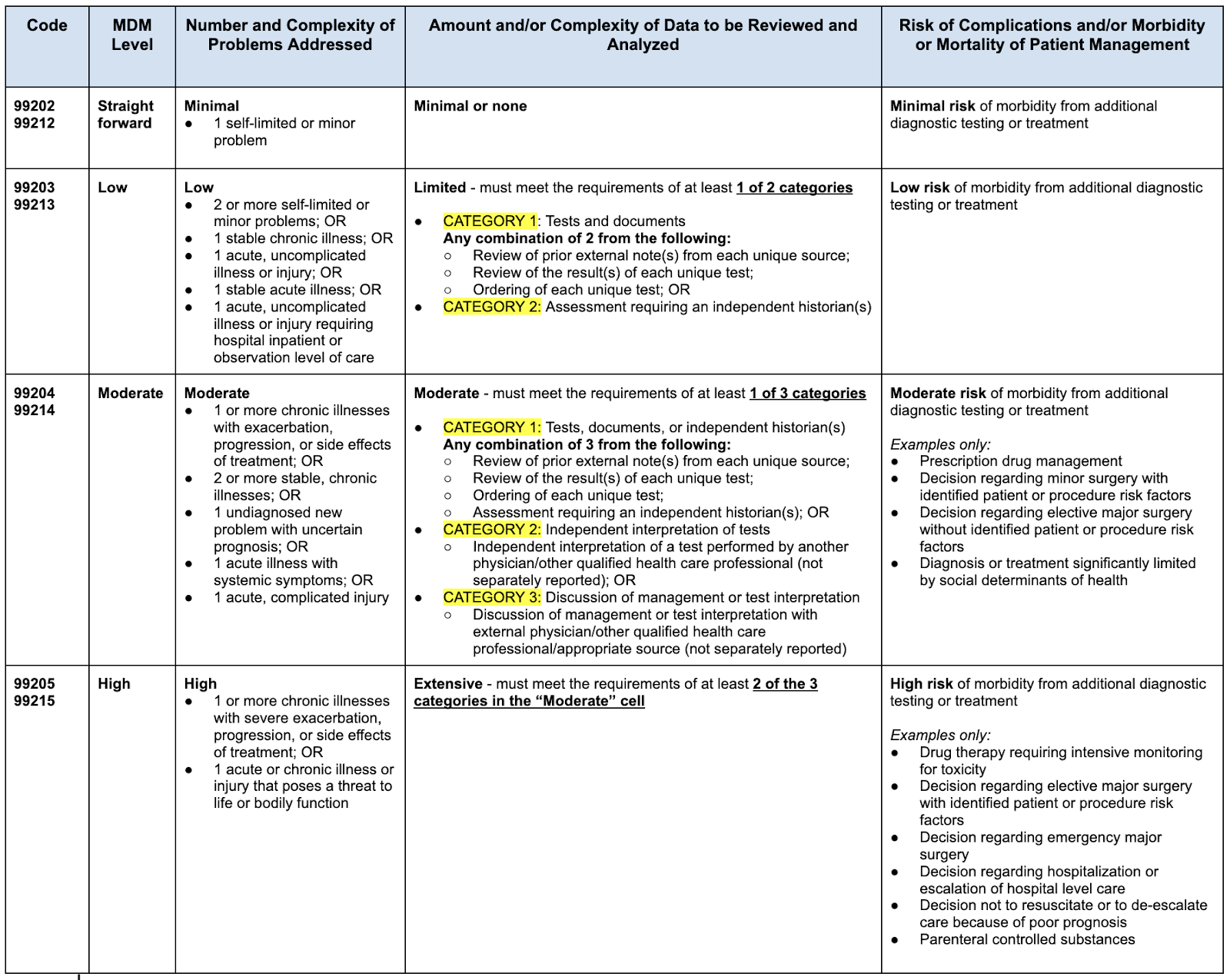
Mdm Solution Comparison Chart
https://eyepegasus.com/wp-content/uploads/2023/05/mdm-scoring-system-summary.png
Oct 1 2014 nbsp 0183 32 Licensed pharmaceutical products marketing authorization should be manufactured only by licensed manufacturers holders of a manufacturing authorization whose Jul 30 2025 nbsp 0183 32 To summarise the draft of the new Chapter 4 answers many questions with regard to modern technologies outsourcing and data integrity but also includes increased
[desc-10] [desc-11]
Gmp Vector Art Icons And Graphics For Free Download
https://static.vecteezy.com/system/resources/previews/002/424/316/original/gmp-good-manufacturing-practice-6-heading-of-infographic-template-with-sample-text-free-vector.jpg
Mexico GMP 2025 Key Updates RegASK
https://regask.com/wp-content/uploads/2025/04/cofepris-mexico-publishes-guidelines-gmp-documentation-active-ingredients-medicines-medical-devices-submissions.png
Gmp Documentation Guidelines - Apr 20 2024 nbsp 0183 32 Understanding implementing and enforcing good documentation practices guidelines is of the utmost importance for pharmaceutical manufacturers So keep reading as