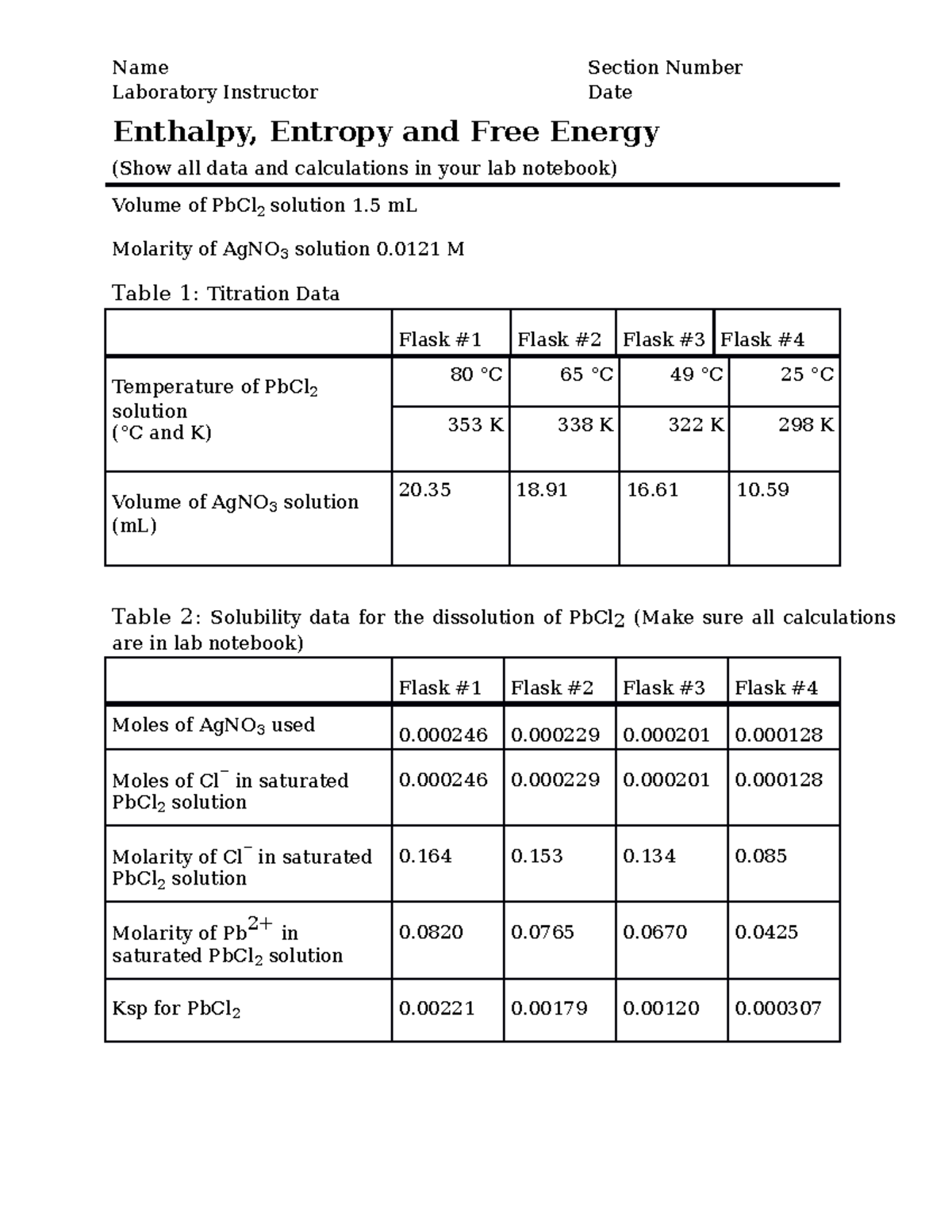
Entropy And Free Energy Worksheet Answers Worksheet on Entropy and Free Energy 1 Define entropy in your own words and list the variables or conditions that you must consider when comparing the entropy of two substances
Key Worksheet 19 Spontaneity Entropy and Free Energy Free Energy Objectives To understand and apply the concept of free energy with respect to equilibria spontaneity and work Free The entropy of a system at 337 1 K increases by 221 7 J mol K The free energy value is found to be 717 5 kJ mol Calculate the change in enthalpy of this system
Entropy And Free Energy Worksheet Answers

Entropy And Free Energy Worksheet Answers
https://i2.wp.com/media.cheggcdn.com/study/42f/42f5f06e-6537-4dcd-bdf6-38b497501b1d/image.png
36 Gibbs Free Energy Worksheet Support Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/36046c45e5377cb580a8df8f4a7d26fa/thumb_1200_1553.png

Entropy Equation Calculations Lesson Study
https://study.com/cimages/videopreview/entropy_v1_135684.jpg
Calculate the entropy change for the following reactions using your thermodynamic properties table 1 N 2 g 3H 2 g 2 NH 3 g 2 H 2 O l H 2 O g Define Gibbs Free Energy Negative Calculate the standard enthalpy change entropy change and free energy change at 2980C for each of the following reactions by using data in the Appendix in the back of your book
Entropy and Gibbs Free Energy Entropy 1 Calculate the change in entropy of a large vat of molten copper when 50 J of energy is removed reversible from it as heat at 1100 176 C 2 Define entropy in your own words and list the variables or conditions that you must consider when comparing the entropy of two substances or when trying to determine the relative
More picture related to Entropy And Free Energy Worksheet Answers
Revision Quiz Entropy And Free Energy Attempt Review Started On
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/bb957a68ecede58a797b5d6b3a406560/thumb_1200_926.png
36 Gibbs Free Energy Worksheet Support Worksheet
https://image2.slideserve.com/4666891/relating-enthalpy-and-entropy-to-spontaneity-l.jpg

Entropy And Free Energy Day 1 Full Notes CHEMISTRY 1002 General
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/34980b68e33b201d14d2f2a6955db99b/thumb_1200_927.png
Dec 20 2019 nbsp 0183 32 Chemists use three energy terms enthalpy entropy and free energy to help them make predictions about whether reactions may take place a The table below shows five 20 Entropy and Free Energy PRACTICE TEST 1 Which of the following represents an increase in entropy a freezing of water b boiling of water c crystallization of salt from a
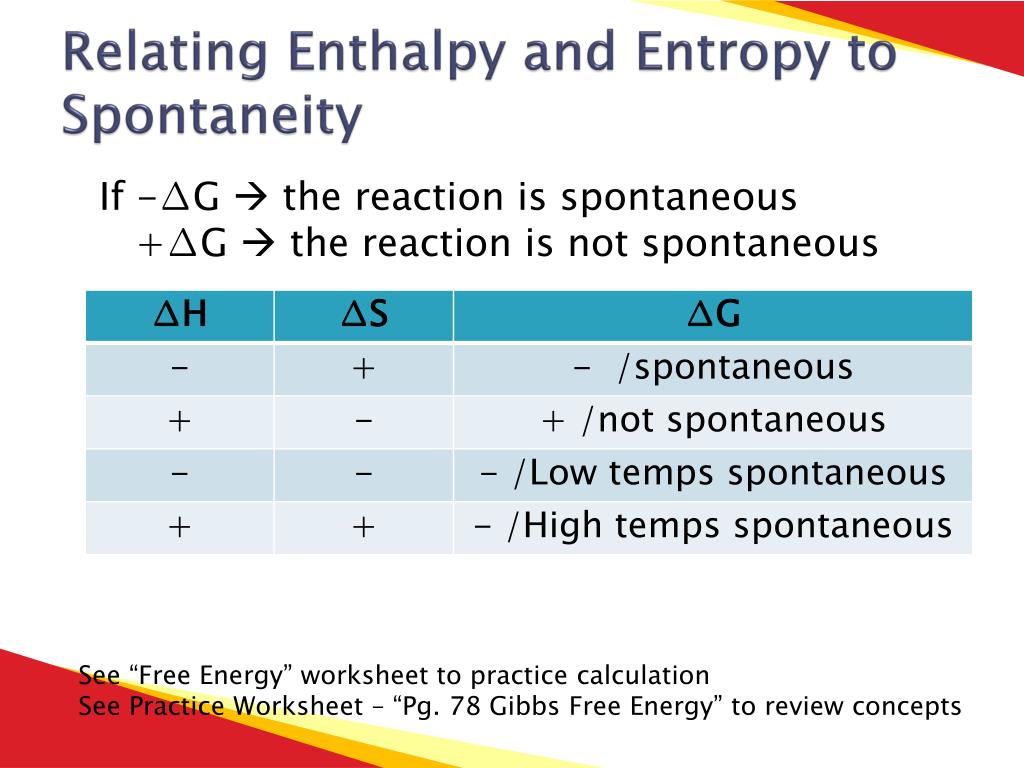
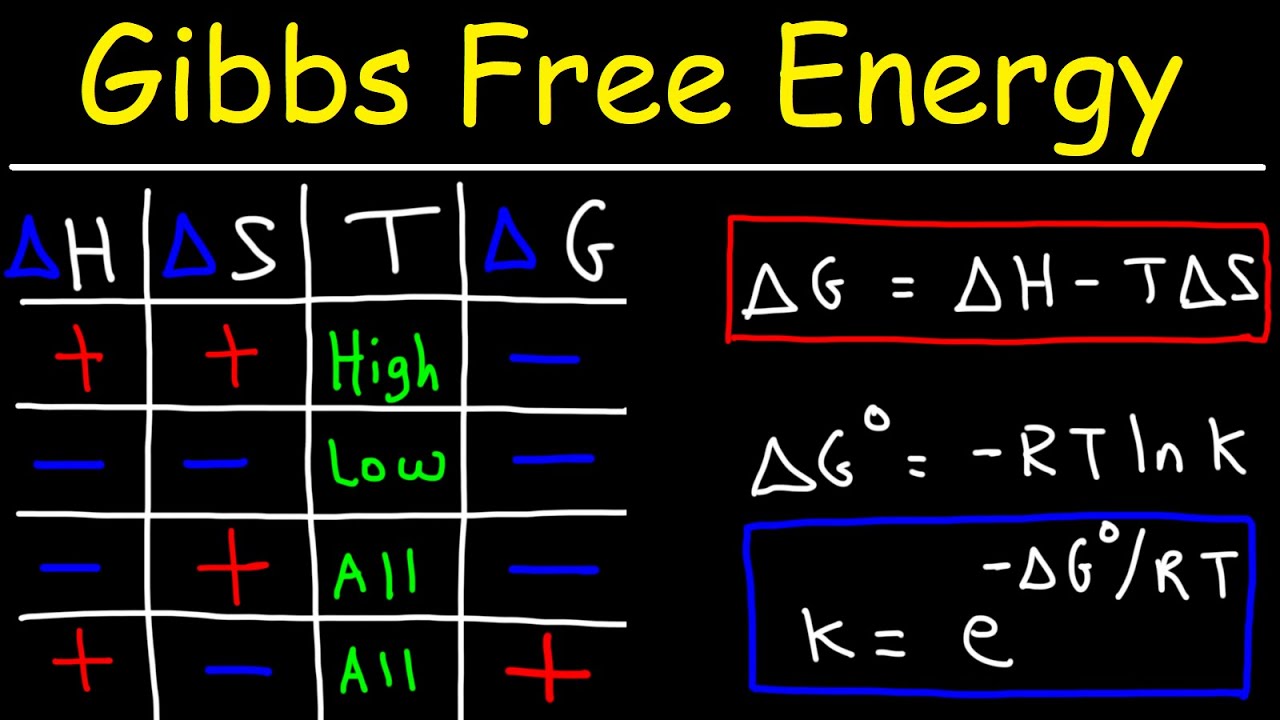
Answers 1 Gibbs free energy combines entropy and enthalpy 2 H is enthalpy a measure of heat S is entropy a measure of disorder T is temperature 3 Spontaneous processes have Worksheet on entropy Gibbs free energy predicting entropy changes and calculating Gibbs free energy Ideal for high school or early college chemistry

L Thuy t M i L V Entropy C Th Gi i Quy t C c V n V Thi t K
https://sciencenotes.org/wp-content/uploads/2021/11/What-Is-Entropy-Definition.png
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K YouTube
https://i.ytimg.com/vi/2KuNzB0cZL4/maxresdefault.jpg
Entropy And Free Energy Worksheet Answers - Apr 21 2021 nbsp 0183 32 Enhanced Document Preview Chem 128 Dr Abel Worksheet on Entropy and Free Energy 1 Define entropy in your own words and list the variables or conditions that you must