Energy And Reaction Rates Pdf Rate of reaction The rate of a reaction is expressed as the change in concentration of a reactant or product per unit time There are several factors which affect whether or not particles will
Reaction Rates and Equilibrium CHEMICAL REACTIONS MATTER AND ENERGY For students using the Foundation edition 18 1 Rates of Reaction assign problem 4 9 11 12 14 and 18 The solution is actually very simple the reaction rate is defined as the rate of change of the concentration of a reactant or product divided by its stochiometric coefficient For the above
Energy And Reaction Rates Pdf
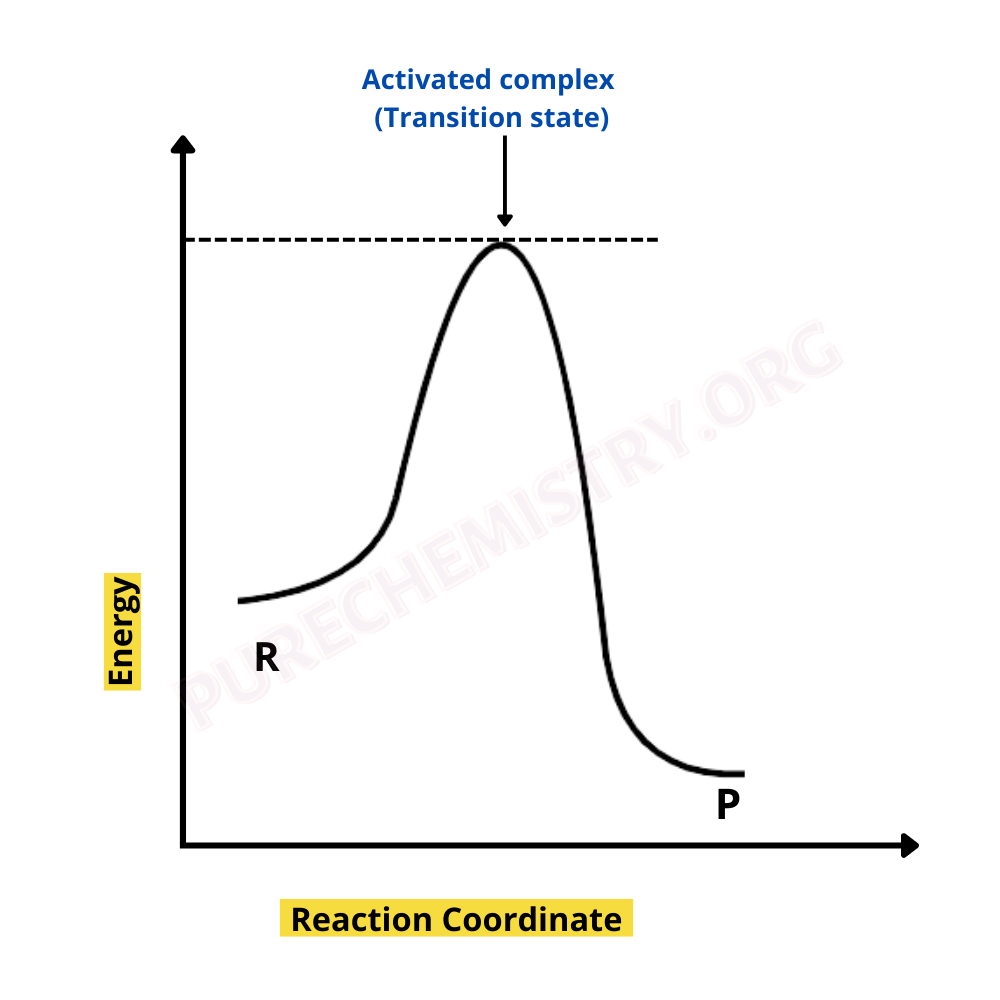
Energy And Reaction Rates Pdf
https://www.purechemistry.org/wp-content/uploads/2022/10/ACTIVATED-COMPLEX-THEORY-ACT-OF-REACTION-RATE.png

Collision Theory And Reaction Rates MME
https://mmerevise.co.uk/wp-content/uploads/2022/10/Collision-theory-1536x1156.png

Reaction Rates And Potential Energy Diagrams YouTube
https://i.ytimg.com/vi/raWoXAw0aX4/maxresdefault.jpg
This chapter introduces you to thermochemistry1 a branch of chemistry that describes the energy changes that occur during chemical reactions In some situations the energy produced by 9 1 9 2 Rate of a reaction For a general reaction of the type A 3B 2Y the rates of consumption of A and B and the rate of formation of Y are defined as follows
Reaction Rate Law For a chemical reaction aA bB cC dD The Rate Law for the forward reaction has the form Rate k A a B b k the reaction rate constant the Theories of Reaction Rates CY1001 2010 T Pradeep There are two basic theories Collision theory and activated complex theory transition state theory Simplest is the collision theory
More picture related to Energy And Reaction Rates Pdf

Reaction Rate Definition Formula And Factors Affecting It
https://www.chemistrylearner.com/wp-content/uploads/2022/11/Reaction-Rate.jpg

Rates Of Reaction And Energy Changes Exothermic Reactions Endothermic
https://i.pinimg.com/originals/fc/83/c7/fc83c7157aea150a93fcc8aec9e50c76.png
CA Lesson 2 Factors Affecting Reaction Rates PDF Catalysis
https://imgv2-1-f.scribdassets.com/img/document/625309409/original/e6a791a50e/1696942660?v=1
Rate of reaction In a chemical reaction the substances that are undergoing the reaction are called the reactants while the substances that form as a result of the reaction are called the The rate of a chemical reaction is the change in the amount of a reactant or product of the reaction with time The rates of chemical reactions differ enormously depending on the reaction
Since the probability of a molecule reacting increases the rate increases Arhenius discovered that most reaction rate data obeyed an equation based on three factors The number of You can use simple collision theory to begin to understand why factors such as concentration affect reaction rate If a collision is necessary for a reaction to occur then it makes sense that
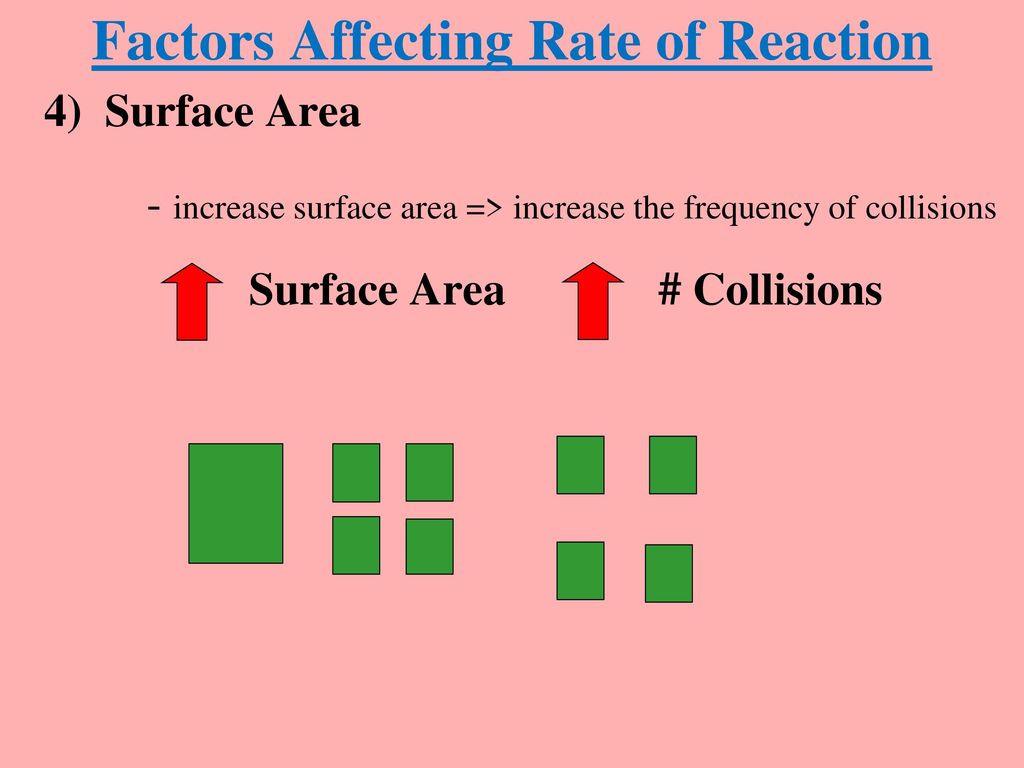
Kinetics Lesson Ppt Download
https://slideplayer.com/slide/12708328/76/images/13/Factors+Affecting+Rate+of+Reaction.jpg

Collision Theory And Reaction Rates MME
https://mmerevise.co.uk/app/uploads/2022/10/MB-Dist-Q-1024x657.png
Energy And Reaction Rates Pdf - 9 1 9 2 Rate of a reaction For a general reaction of the type A 3B 2Y the rates of consumption of A and B and the rate of formation of Y are defined as follows
