Calculating Heat And Specific Heat Worksheet 3 Calculate the energy of combustion for one mole of butane if burning a 0 367 g sample of butane C 4 H 10 has increased the temperature of a bomb calorimeter by 7 73 C The heat capacity of the bomb calorimeter is 2 36 kJ C answer This content is available to registered users only
Calculating Heat and Specific Heat Heat q q c x m x T Specific Heat c c q m x T Variable Symbol Unit Heat q J Specific heat c J g 0C mass m g Change in temperature T 0C Specific Heat Substance J g oC Air 1 01 Aluminum 0 902 Copper 0 385 Gold 0 129 Iron 0 450 Mercury 0 140 NaCl 0 864 Ice 2 03 Answers mc T where Q heat energy m mass and T change in temp Remember T Tfinal Tinitial Show all work and proper units A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 C to 175 C Calculate the specific heat capacity of iron How many joules of heat are needed
Calculating Heat And Specific Heat Worksheet

Calculating Heat And Specific Heat Worksheet
https://s3.studylib.net/store/data/008947763_1-dca3e2c850eb7c7ce2a31f670b8b2bc6.png
Calculating Specific Heat Worksheet
https://imgv2-1-f.scribdassets.com/img/document/392411455/original/0624990a6f/1610279825?vu003d1

50 Calculating Specific Heat Worksheet Chessmuseum Template Library
https://chessmuseum.org/wp-content/uploads/2019/10/calculating-specific-heat-worksheet-fresh-specific-heat-worksheet-of-calculating-specific-heat-worksheet.jpg
Worksheet Calculations involving Specific Heat 1 For q m c T identify each variables by name the units associated with it 2 Heat is not the same as temperature yet they are related Explain how they differ from each other a Perform calculations using q m c T b Answers to Worksheet 17 Calculating Heat The specific heat capacity c of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K Units are in J g K or J g C The molar heat capacity C of a substance is the amount of heat required to raise the temperature of
Calculate heat specific heat mass and change in temperature of a reaction Name 9 www mrpalermo 4 When a sample of 25 0 g of water is cooled from 20 0 C to 10 0 C what is the number of Joules of energy released 5 A sample of water is heated from 10 0 C to 15 0 C by adding 125 58 Joules of heat Specific Heat Worksheet Name in ink C q mAT where q heat energy m mass and T temperature Remember AT Tfinal Tinitial Show all work and proper units Answers are provided at the end of the worksheet without units 1 A 15 75 g piece of iron sorbs 1086 75 joules of heat energy and its temperature changes from 25 0 1750C
More picture related to Calculating Heat And Specific Heat Worksheet

50 Calculating Specific Heat Worksheet Chessmuseum Template Library
https://chessmuseum.org/wp-content/uploads/2019/10/calculating-specific-heat-worksheet-inspirational-calculating-specific-heat-worksheet-answers-of-calculating-specific-heat-worksheet.jpg
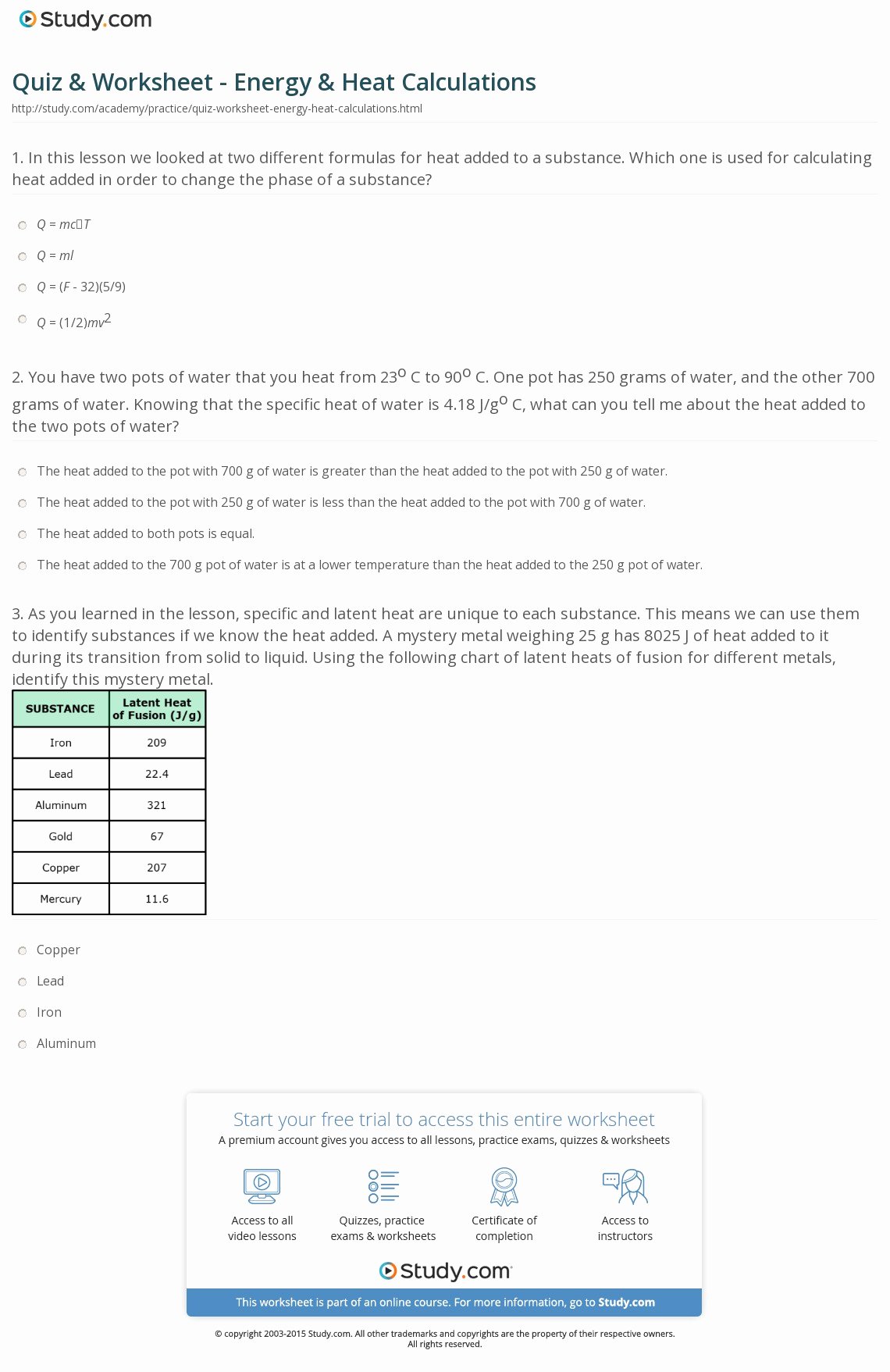
50 Calculating Specific Heat Worksheet Chessmuseum Template Library
https://chessmuseum.org/wp-content/uploads/2019/10/calculating-specific-heat-worksheet-elegant-quiz-amp-worksheet-energy-amp-heat-calculations-of-calculating-specific-heat-worksheet.jpg

Calculating Heat And Specific Heat Worksheet Answers Worksheets
https://static.docsity.com/documents_first_pages/2021/04/20/677f994510877a7c683793f2196badd3.png
The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled The equation that relates heat q q to specific heat cp c p mass m m and temperature change T T is shown below q cp m T q c p m T Specific heat capacity c can be experimentally determined by measuring the temperature change T Tf Ti in C that a known mass m undergoes when it loses or gains a quantity of heat q so it follows that q m c T T Recall that the density of pure water 1 00 g mL 1 so that for water 100 g 100 cm3 No Question
Calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 500 joules of heat and its temperature changes from 32 C to 57 C 4 100 0 g of 4 0 C water is heated until its temperature is 37 C If the specific heat of water is 4 18 J g C calculate the amount of heat energy needed to cause this rise in Specific Heat Capacity 900 J kg o C Temperature change 0 100 3 Oh no There is a temperature change so work out what the difference is super hard right Mass 4 kg Specific Heat Capacity 900 J kg o C Temperature change 100 0 100 o C

Calculating Specific Heat Worksheet
https://db-excel.com/wp-content/uploads/2019/09/specific-heat-capacity-worksheet-key-engineering-studocu-2.png
Calculating Specific Heat Extra Practice Worksheet Printable Word
https://i2.wp.com/media.cheggcdn.com/media/ad7/ad7b1026-a16c-4a3a-b1fc-26aca3a3f122/image
Calculating Heat And Specific Heat Worksheet - Calculate heat specific heat mass and change in temperature of a reaction Name 9 www mrpalermo 4 When a sample of 25 0 g of water is cooled from 20 0 C to 10 0 C what is the number of Joules of energy released 5 A sample of water is heated from 10 0 C to 15 0 C by adding 125 58 Joules of heat

