Empirical Molecular Formula Practice Worksheet Answer Key Pdf Worksheet 8 Empirical Formulas 1 State the empirical formula for each of the following compounds a C 4H8 b C 2H6O2 c N2O5 d Ba 3 PO 4 2 e Te 4I16 2 What is the empirical formula for a compound that contains 0 063 mol chlorine and 0 22 mol oxygen 3 What is the empirical formula for a compound that contains 26 1 carbon 4 3 hydrogen
Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula Empirical Formulas Molecular Formulas EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been provided 2 Express these quantities in moles 3 Divide the number of moles by the minimum number of moles for each element
Empirical Molecular Formula Practice Worksheet Answer Key Pdf
Empirical Molecular Formula Practice Worksheet Answer Key Pdf
https://media.cheggcdn.com/study/06d/06d3e824-b032-432d-b735-42d1c4017b3a/image

Empirical Formula Definition Examples Video Lesson Transcript
https://study.com/cimages/videopreview/uso1zfhp27.jpg

Empirical molecular Formula Practice Worksheet Answer Key Printable
https://chessmuseum.org/wp-content/uploads/2019/10/empirical-and-molecular-formulas-worksheet-fresh-empirical-formula-worksheets-answer-key-of-empirical-and-molecular-formulas-worksheet.jpg
Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula 5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m ol
Show your work for the calculation of empirical formula here Excessive physical activity lactic acid molecular mass 90 08 g per mole forms in muscle tissues and is responsible for muscle soreness Elemental analysis shows that this compound has 40 0 carbon 6 71 hydrogen and 53 3 oxygen Determine the empirical formula of lactic acid Empirical and Molecular Formulas Worksheet 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O 3 What is empirical formula if compound consists of 21 2 N 6 1 H 24 2 S and 48 5 O
More picture related to Empirical Molecular Formula Practice Worksheet Answer Key Pdf

Calculating Empirical And Molecular Formula YouTube
https://i.ytimg.com/vi/cYvdlvwJ8tM/maxresdefault.jpg

Molecular Vs Empirical Formula Slideshare
https://image1.slideserve.com/3095492/empirical-and-molecular-formula-notes-n.jpg

Empirical And Molecular Formula Worksheet Printable Pdf Download
https://data.formsbank.com/pdf_docs_html/88/884/88467/page_1_thumb_big.png
Empirical formula worksheet To print or download this file click the link below Empirical Formula Worksheet pdf PDF document 1 83 MB 1921394 bytes Empirical Formula Worksheet Empirical Formula a formula showing the smallest whole number mole ratio 1 Convert the grams of each element to moles 2 To find the simplest ratio divide each element s mole value by the smallest mole value This will ensure a something to 1 ratio 3 If the mole ratios are NOT all whole numbers you must
Determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g mol cCQ 12 01 B MA 34 937 a 53 35 14 A compound has a molar mass of 86 g mol and has the percent composition by mass of 55 8 C 37 2 O and 7 0 H Determine the empirical formula and the molecular formula 7 H 233 2 33 Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound 7 A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole
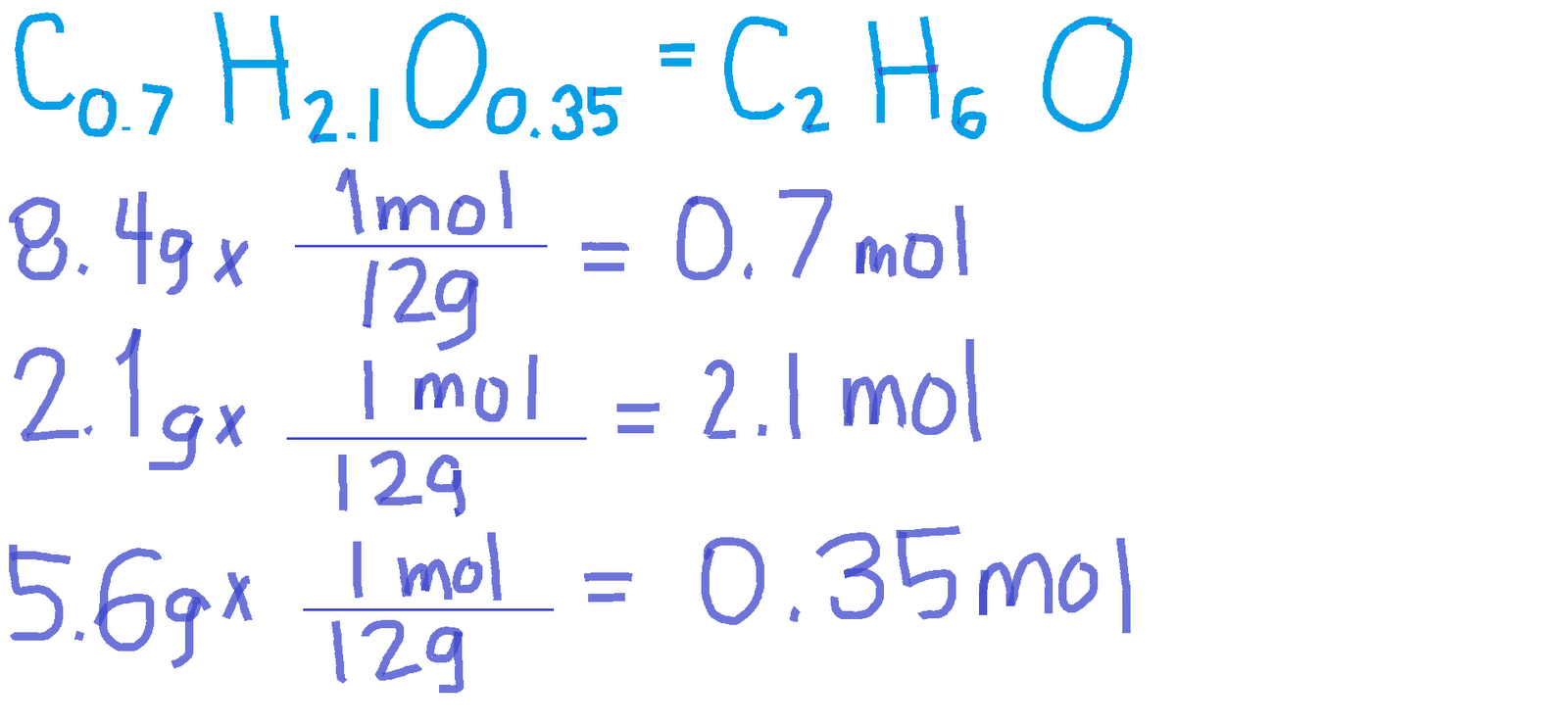
CHEMISTRY 11 EMPIRICAL MOLECULAR FORMULAS
http://1.bp.blogspot.com/_z9emnNOdBIE/TT5ZytnFkxI/AAAAAAAAA2E/-vFCJM0GHqs/s1600/example+empirical.png

Empirical Molecular Formula Practice
https://s2.studylib.net/store/data/009851471_1-ce76bbd45a625175224d54a7ec60a84a-768x994.png
Empirical Molecular Formula Practice Worksheet Answer Key Pdf - 5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m ol
