Dalton S Law Of Partial Pressure Worksheet Answers What is the partial pressure of nitrogen if the total pressure is 99 42 kPa Solution 1 The vapor pressure of water at 40 0 176 C is looked up and found to be 7 38 kPa 2 Dalton s Law of Partial Pressure is used P tot P N 2 P H 2 O 99 42 kPa P N 2 7 38 kPa P N 2 92 04 kPa
The partial pressure of Ne if Ar is present in a mole fraction of 0 34 Step 1 Calculate the mole fraction of Ne x Ne 1 x Ar x Ne 1 0 34 x Ne 0 66 Step 2 Use Dalton s Law of Partial Pressures to calculate the partial pressure of Ne P Ne P Tot x Ne P Ne 1 5 atm 0 66 P Ne 0 99 atm Correct answer 0 99 atm The pressure each gas exerts in mixture is called its partial pressure Here is an example A gas mixture contains hydrogn helium neon and argon The total pressure of the mixture is 93 6 kPa The partial pressures of helium neon and argon are
Dalton S Law Of Partial Pressure Worksheet Answers
Dalton S Law Of Partial Pressure Worksheet Answers
https://lh5.googleusercontent.com/28zFaXFDRgBYnG6ybBTj7ZDRmXd_akvkbWfc3wA0BNg_oXSuYWsr80mePg=w1200-h630-p

Solved Dalton s Law Of Partial Pressures Worksheet 1 Blast Chegg
https://media.cheggcdn.com/media/69c/69c093b5-469e-49d3-bfd5-2c3cf1f110d8/image.png

Daltons Law Lesson Plans Worksheets Reviewed By Teachers
https://content.lessonplanet.com/resources/thumbnails/215140/original/nze3mjmylmpwzw.jpg?1414305754
Dalton s Law says that the sum of the individual pressures of all the gases that make up a mixture is equal to the total pressure or PT P1 P2 P3 The partial pressure of each gas is equal to the mole fraction of each gas times the total pressure Dalton s Law of Partial Pressures Worksheet Name 1 Blast furnaces give off many unpleasant and unhealthy gases If the total air pressure is 0 99 atm the partial pressure of carbon dioxide is 0 05 atm and the partial pressure of hydrogen sulfide is 0 02 atm what is the partial pressure of the remaining air 2
1 Define Dalton s Law in your own words and number of moles True or False Explain 2 Different types of gases exert different pressures on their containers even if they have the same volume temperature 3 Write a generic equation for determining the pressure of Dalton s Law of Partial Pressure 1 A metal tank contains three gases oxygen helium and nitrogen If the partial pressures of the three gases in the tank are 35 atm of O 2 5 atm of N 2 and 25 atm of He what is the total pressure inside of the tank 2 Blast furnaces give off many unpleasant and unhealthy gases
More picture related to Dalton S Law Of Partial Pressure Worksheet Answers

Dalton s Law Of Partial Pressure Worksheet Answers Printable Word
https://i2.wp.com/i.ytimg.com/vi/r-4KZFpL4q4/maxresdefault.jpg

CHEMISTRY 101 Dalton s Law Of Partial Pressures And Mole Fraction
https://i.ytimg.com/vi/8naLOENwagE/maxresdefault.jpg
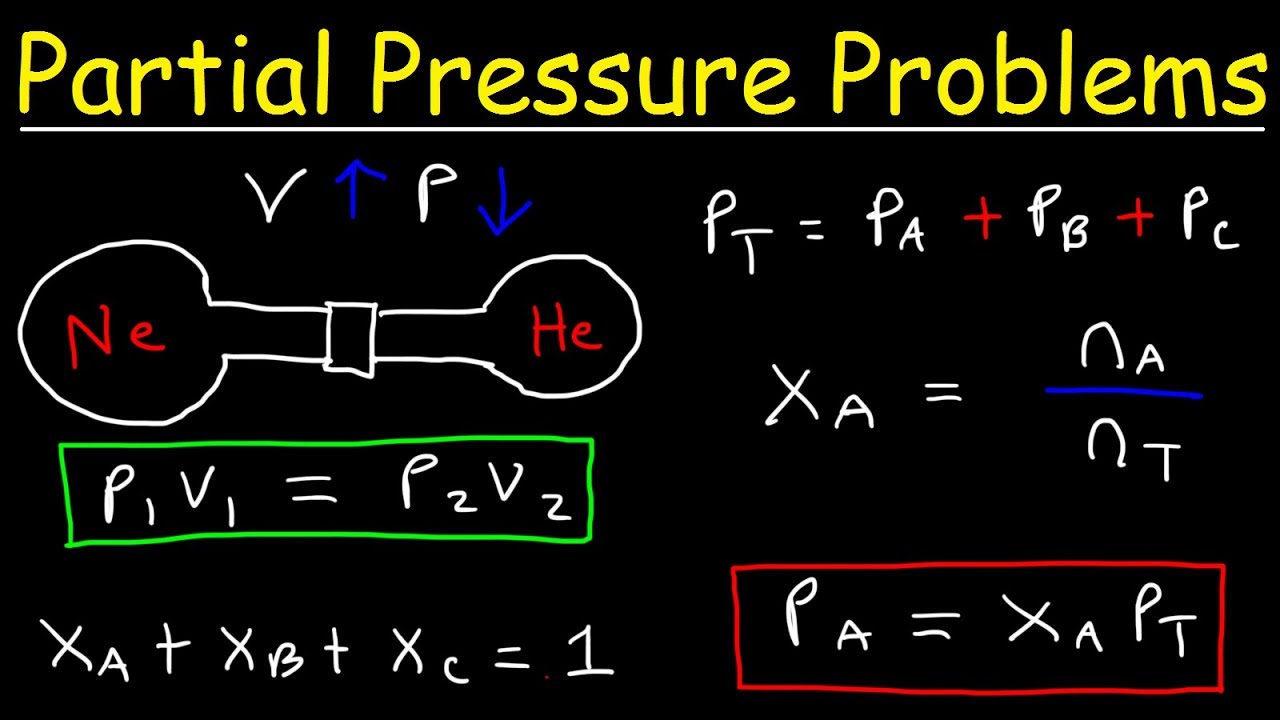
Dalton s Law Of Partial Pressure Problems Mole Fraction Chemistry Gas
https://i.ytimg.com/vi/J7YRwU7IV8Q/maxresdefault.jpg
Dalton s Law of Partial Pressure Worksheet Dalton s Law says that the sum of the individual pressures of all the gases that make up a mixture is equal to the total pressure or Ptotal P1 P2 P3 Dalton s Law Of Partial Pressure Problems 1 The volume of hydrogen collected over water is 453 mL at 18 176 C and 780 mm Hg What is its volume dry at STP 2 A 423 mL sample of dry oxygen at STP is transferred to a container over water at 22 176 C and 738 mm Hg What is the new volume of the oxygen
Dalton s Law of Partial Pressures Answers 1 If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C what will the pressure of the resulting mixture of gases be Using the ideal gas law you can determine that the partial pressure of nitrogen in this mixture will be 2 09 atm 211 8 kPa and the partial What is Dalton s Law Dalton s law of partial pressures is a gas law which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture

Dalton s Law Of Partial Pressure Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png

DALTON S LAW OF PARTIAL PRESSURE Storyboard By 85dadb25
https://sbt.blob.core.windows.net/storyboards/85dadb25/dalton-s-law-of-partial-pressure.png?utc=132875125861300000
Dalton S Law Of Partial Pressure Worksheet Answers - Are you struggling to fully understand Dalton s Law of Partial Pressure This worksheet is designed to help students break down the concept into manageable steps ensuring a solid grasp of this essential topic in chemistry