Combined Gas Law Worksheet Answers Solve the following problems As always include enough work and show the units to ensure full credit 1 The pressure of a gas changes from 120 kPa to 50 kPa The volume changes from 45 L to 40 L
Use the combined gas law to solve the following problems 1 If I initially have a gas at a pressure of 12 atm a volume of 23 liters and a temperature of 200 K and then I raise the pressure to 14 atm and increase the temperature to 300 K what is the new volume of the gas 2 A gas takes up a volume of 17 liters has a pressure of 2 3 atm Combined Gas Law Worksheet Solutions 1 If I initially have 4 0 L of a gas at a pressure of 1 1 atm what will the volume be if I increase the pressure to 3 4 atm 1 1 atm 4 0 L 3 4 atm x L x 1 29 L 2 A toy balloon has an internal pressure of 1 05 atm and a volume of 5 0 L
Combined Gas Law Worksheet Answers
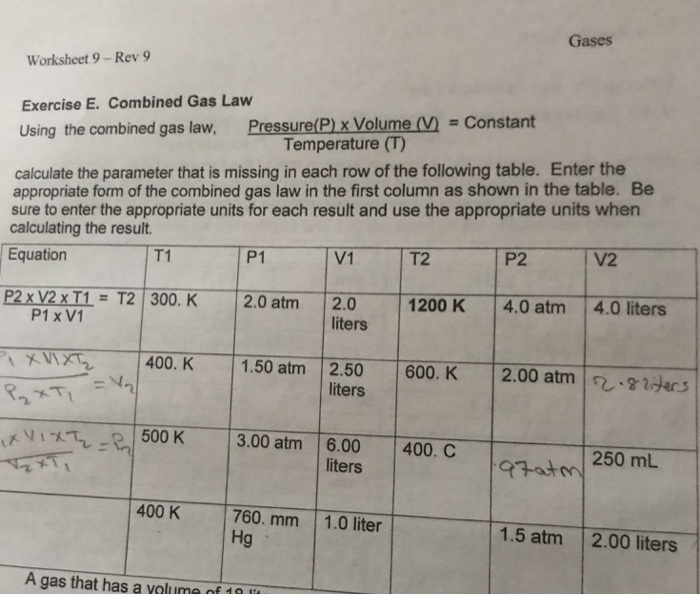
Combined Gas Law Worksheet Answers
https://media.cheggcdn.com/media/a28/a28336cc-93df-4f57-a10b-ac6ef5a93918/image.png
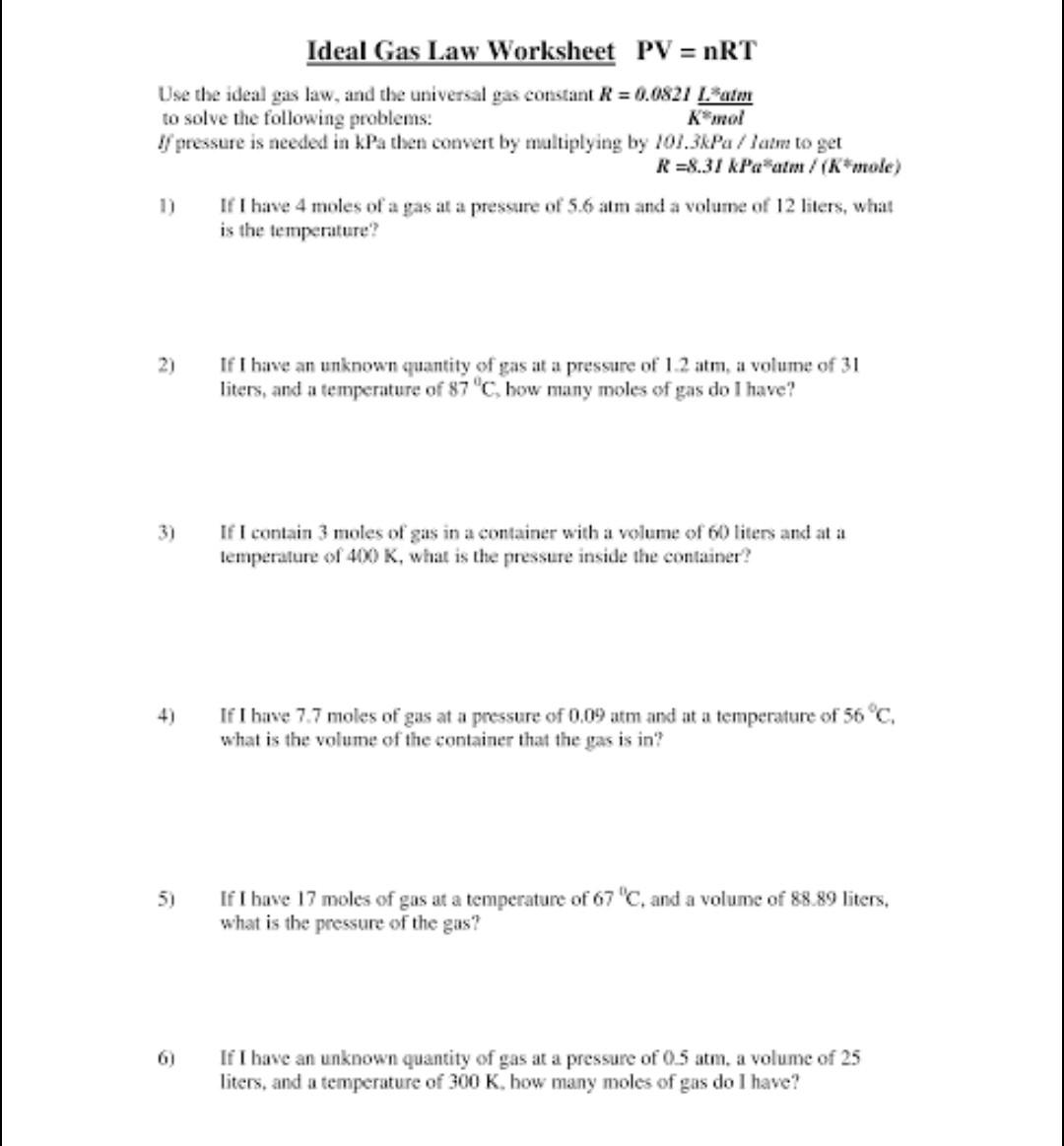
Get Answer Ideal Gas Law Worksheet PV NRT Use The Ideal Gas Law
https://files.transtutors.com/book/qimg/55f8661b-b91b-4b84-a0af-3c4374ac195e.png
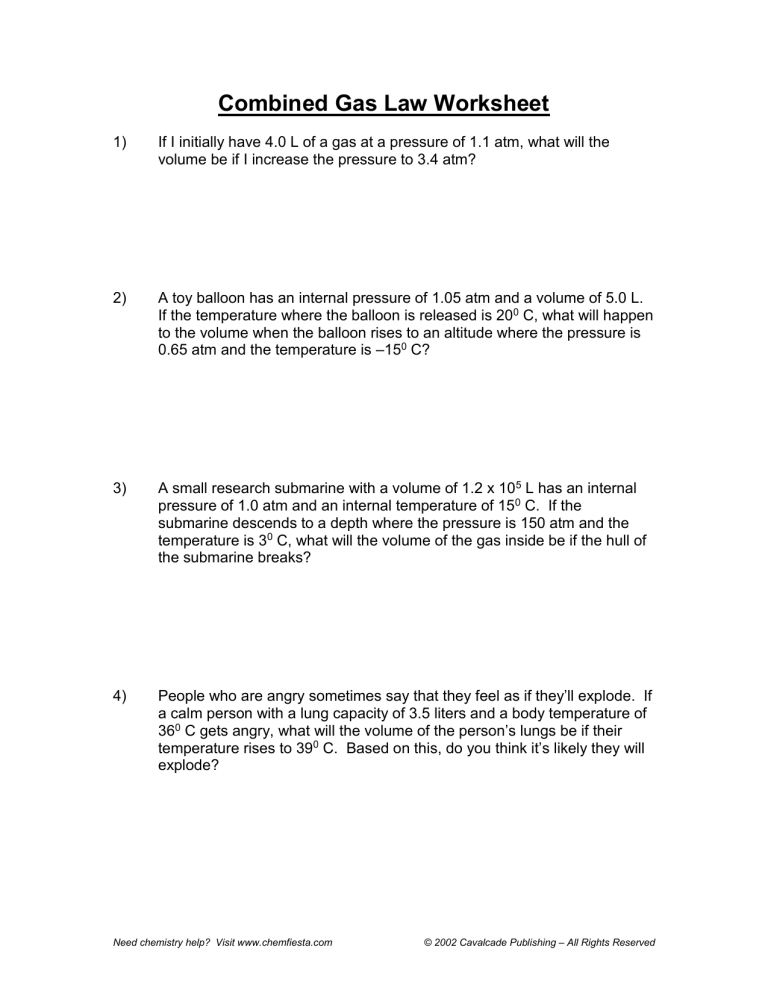
Combined Gas Law Worksheet 1
https://s3.studylib.net/store/data/025427748_1-fa3270c544423eb4869bef7cd74bc2b9-768x994.png
Oct 30 2020 nbsp 0183 32 You have a sample of gas with a pressure of 1 86 atm volume of 4 33 L and temperature of 26 5 176 C If you cool it to 12 7 176 C and decrease the volume to 3 45 L what will the pressure be Answer Worksheet Combined Gas Law 1 A gas has a volume of 800 0 mL at minus 23 00 oc and 300 0 torr What would the volume of the gas be at 227 0 oc and 600 0 torr of pressure 2 500 0 liters of a gas are prepared at 700 0 mm Hg and 200 0 oc The gas is placed into a tank under high pressure When the tank cools to 20 0 oc the pressure of the gas is
A gas has a temperature of 14 C and a volume of 4 5 litres If the temperature is raised to 29 C and the pressure does not change what is the new volume of the gas Gas Laws Worksheet 2 Boyle Charles and Combined Gas Laws Due Monday 2 27 Boyle s Law Problems P1V1 P2V2 atm 760 0 mm Hg 101 3 kPa 760 0 torr 1 If 22 5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature What is the new volume
More picture related to Combined Gas Law Worksheet Answers

Gas Laws Notes
https://s3.studylib.net/store/data/006729738_1-70d97170f5a8375a5b1e06308cdcc204.png

Combined Gas Law Worksheet Answers
https://i.pinimg.com/originals/03/11/5e/03115e849663acf997dccb04e13c25b7.jpg
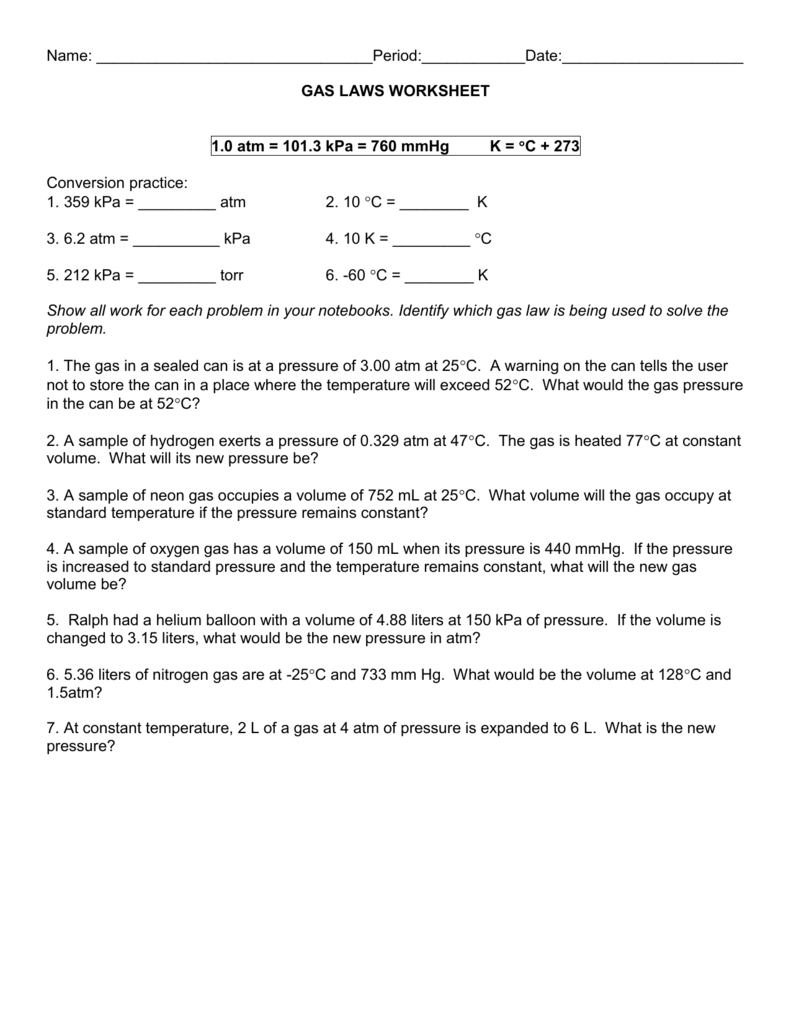
Worksheet Mixed Gas Law Worksheet
https://s3.studylib.net/store/data/009221976_1-66e6457ca396ab87cae6eba038b452e2.png
Step 1 Write the problem solving form of Boyle s Law P 1 V 1 P 2 V 2 Step 2 Multiply by the problem solving form of Charles Law P 1 V 1 V 1 T 1 P 2 V 2 V 2 T 2 P 1 V 12 T 1 P 2 V 22 T 2 Step 3 Multiply by the problem solving form of Gay Lussac s Law P 1 V 12 T 1 P 1 T 1 P 2 V 22 T 2 P 2 T 2 Worksheet Combined Gas Law and Ideal Gas Law Name 1 A 952 cm3 container of gas is exerting a pressure of 108 kPa while at a temperature of 48 176 C Calculate the pressure of this same amount of gas in a 1236 cm3 container at a temperature of 64 176 C 2 At STP a sample of gas occupies 24 5 mL
The following practice problems are to master to topics on the ideal gas laws Boyle s law Charles s law and Avogadro s Law as well as the combined gas law equation There are examples to work on the Dalton law of partial pressures the Graham s law of Gas Laws Worksheet 1 Boyle s Charles Gay Lussac s and combined Gas Laws A Gas Sample Contained in a cylinder equipped with a movable piston occupied 300 0mL at a pressure of 2 00 atm

Post Gas Laws Exploration Worksheet Answers
https://s3.studylib.net/store/data/008991486_1-878570f029005c25e82204000b5ab811.png
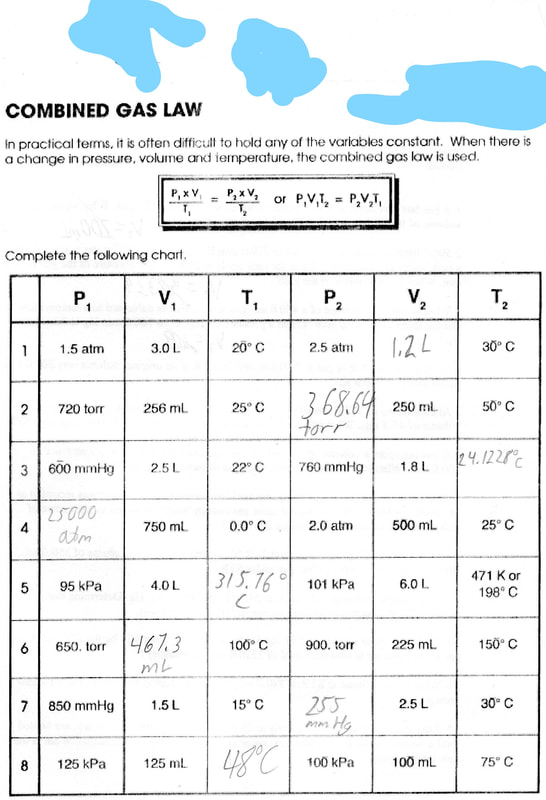
Chemistry Page 2
https://classworkassignmentanswers.weebly.com/uploads/7/9/5/5/79550400/combined-gas-law-li_orig.jpg
Combined Gas Law Worksheet Answers - Mar 13 2023 nbsp 0183 32 Empirical Gas Laws Combined Gas Law Boyle s Law and Charles Law can be combined into one equation that expresses the volume temperature and pressure relationships for a fixed amount of gas