Calculate Rate Of Reaction From Graph To plot a graph to calculate the rate of reaction you need to select two variables such as time and concentration and plot them against each other The slope of the line represents the rate of reaction
Jul 12 2023 nbsp 0183 32 The rates of reaction at a number of points on the graph must be calculated this is done by drawing tangents to the graph and measuring their slopes These values are then tabulated The quickest way to proceed from here is to plot a Investigate factors which affect the speed of a chemical reaction and calculate the time taken for the reaction to occur in National 5 Chemistry
Calculate Rate Of Reaction From Graph

Calculate Rate Of Reaction From Graph
https://i.ytimg.com/vi/MCG19QHBOnw/maxresdefault.jpg
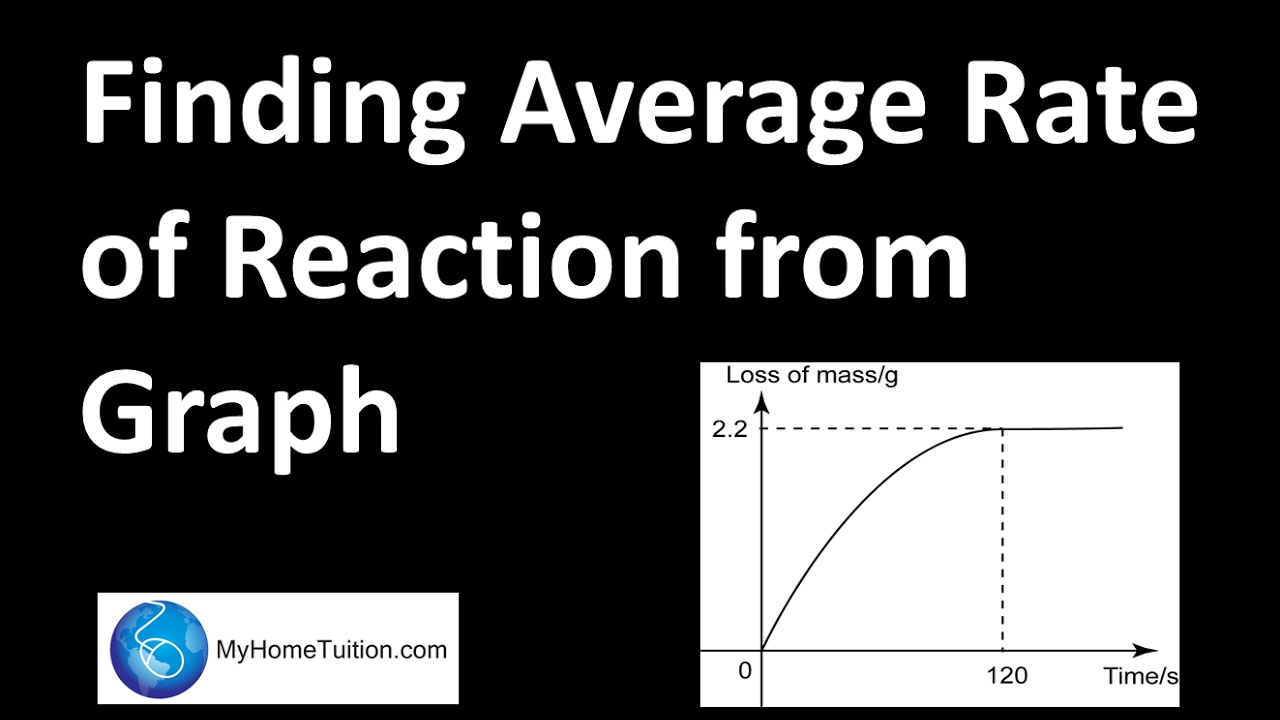
Finding Average Rate Of Reaction From Graph Chemistry YouTube
https://i.ytimg.com/vi/knAjEa4-0KM/maxresdefault.jpg

Rates Of Reaction And Graphs YouTube
https://i.ytimg.com/vi/e6fBAZ36y4s/maxresdefault.jpg
Jul 10 2024 nbsp 0183 32 Calculate the mean rate of reaction between 10 s and 40 s Answer Step 1 Using a ruler draw two lines upwards from the x axis at 10 seconds and 40 seconds Step 2 At the points these lines meet the curve extend two horizontal lines to meet the y axis and read the values Apr 16 2014 nbsp 0183 32 You calculate the rate of reaction from the slope of a graph of concentration vs time Assume we have a reaction 2A 3B By definition rate 1 2 A t 1 3 B t
The rate of reaction can be analysed by plotting a graph of amount of product against time The graph below shows this for two reactions Rate graphs allow us to calculate the rate of a chemical reaction To calculate the rate of reaction from a rate graph we must first draw a tangent to the curve of the graph
More picture related to Calculate Rate Of Reaction From Graph

R2 2 1 Rate Of Reaction YouTube
https://i.ytimg.com/vi/PHp9EwlhrQQ/maxresdefault.jpg

R2 2 1 How Do We Calculate The Rate Of Reaction From A Time
https://i.ytimg.com/vi/diSaZvbVUQ8/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AHKBYAC4AOKAgwIABABGF0gXShdMA8=&rs=AOn4CLABNzS9n7T6iykcuNeKMhUePv-sSA

How To Calculate The Initial Rate Of Reaction From A Graph YouTube
https://i.ytimg.com/vi/A_2OiyEq_bY/maxresdefault.jpg
Nov 21 2023 nbsp 0183 32 To calculate rate of reaction from a graph the general formula change in concentration change in time is used To find the average rate find the change in concentration change We have the rate of reaction graph below Estimate the rate of reaction at 2 minutes The first step in estimating the rate of reaction at 2 minutes is to draw a tangent to the curve at 2 minutes we draw a straight line that touches the curve at 2 minutes
There are two main ways to find the rate of a reaction using the equation above or finding the gradient of a reaction rate graph The gradient slope of the graph tells you how fast the reaction is Rate Graphs and Orders This lesson covers Designing experiments to determine the rate equation The initial rates method and clock reactions Continuous monitoring methods and colorimetry Interpreting rate concentration graphs Calculating rate constants from half life data

Calculate Average Rate And Instantaneous Rate Of Reaction Using Graph
https://i.ytimg.com/vi/Rv_X53Ok3aI/maxresdefault.jpg
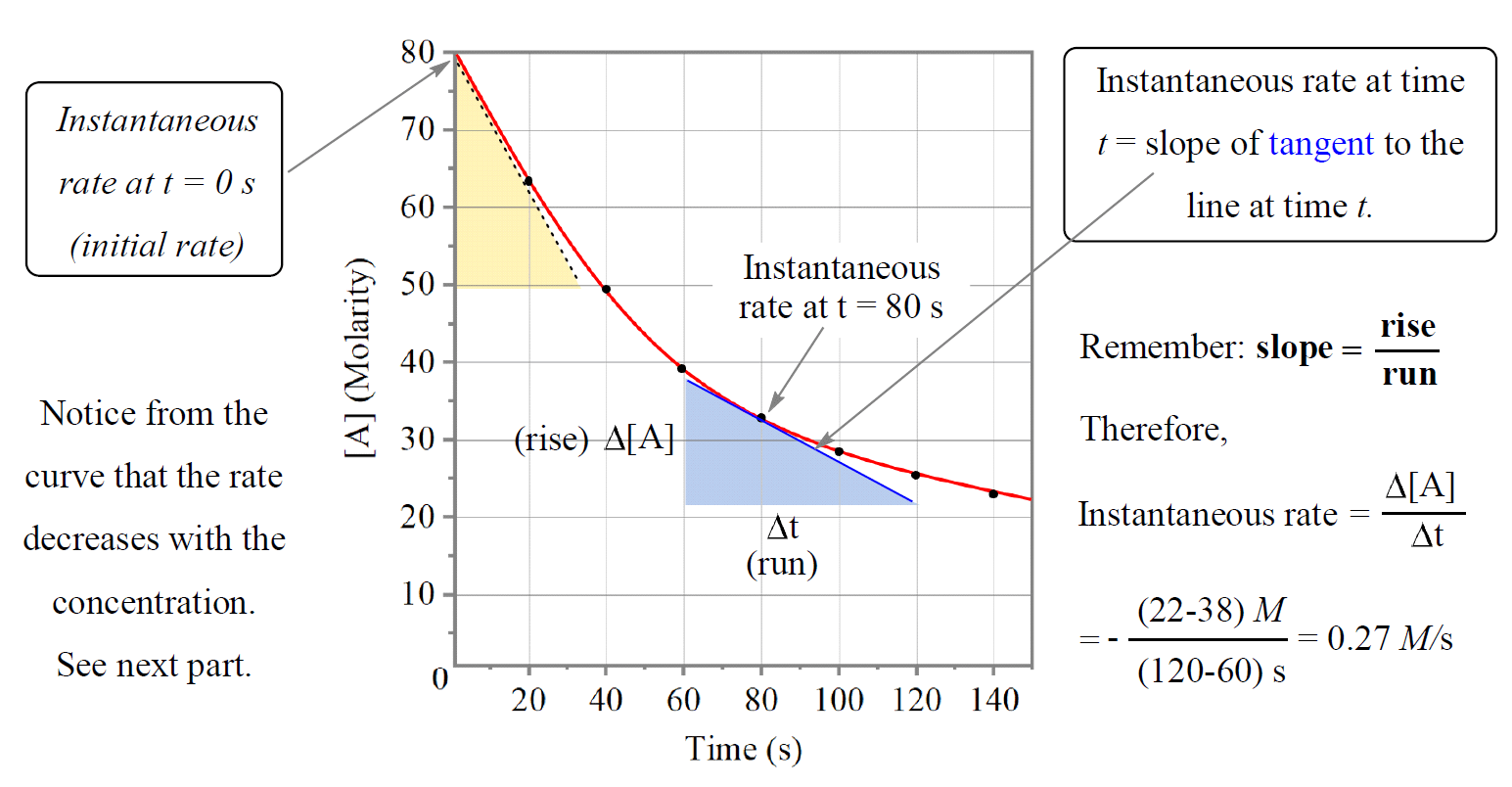
Reaction Rate Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/04/instantaneous-rate-of-reaction-tangent-slope.png
Calculate Rate Of Reaction From Graph - Feb 13 2023 nbsp 0183 32 Plot a graph of the concentration versus t ln concentration versus t and 1 concentration versus t and then determine the rate law and calculate the rate constant