Balancing Redox Equations Redox reactions can be balanced with the half reaction method The half reaction shows the oxidation and reduction processes separately For example Zn Ag Zn2 Ag Follow
In a redox oxidation reduction reaction electrons are transferred between chemical species Balancing these reactions is crucial because both mass and charge must be conserved The Balancing redox reactions allows for the net reaction equation to be conveyed in a concise manner Balance the number of electrons between half reactions so the number lost in one is
Balancing Redox Equations
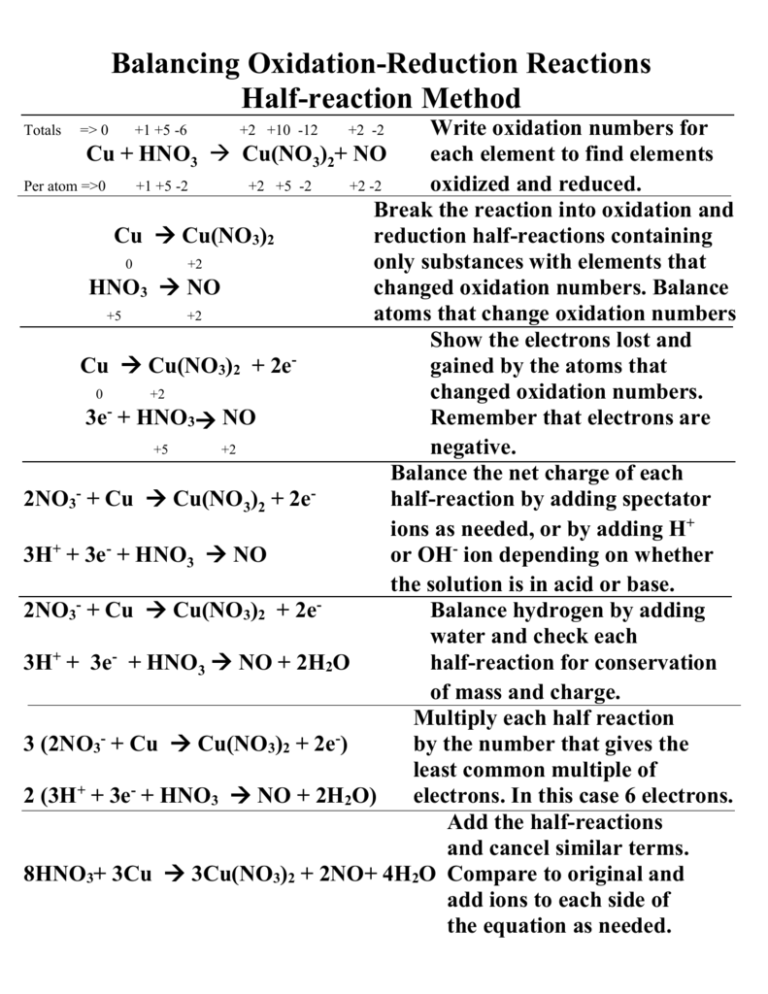
Balancing Redox Equations
https://s3.studylib.net/store/data/007467152_1-03b9e304d0121f0dd0121f00393ec263-768x994.png
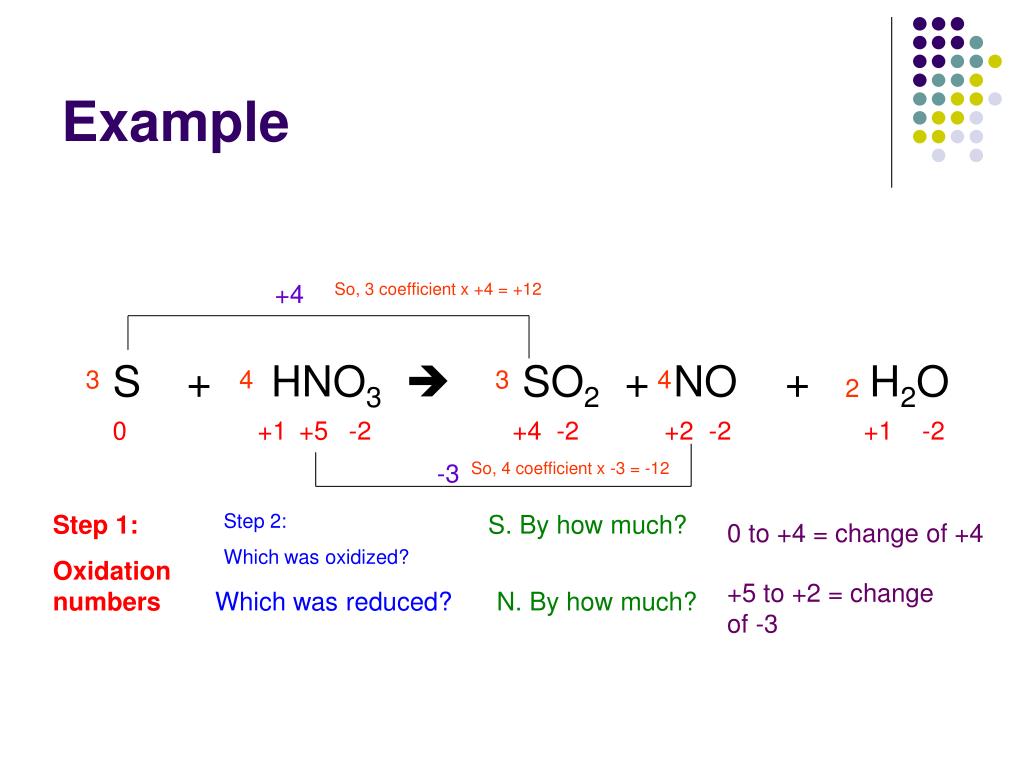
PPT Balancing Redox Equations PowerPoint Presentation Free Download
https://image3.slideserve.com/5431268/example-l.jpg

Writing Balanced Redox Equations Using Half Equations Crunch Chemistry
https://i0.wp.com/crunchchemistry.co.uk/wp-content/uploads/2022/05/OS-4.png?resize=2048%2C1649&ssl=1
One very useful approach is to use the method of half reactions which involves the following steps Write the two half reactions representing the redox process Balance all elements Guidelines for balancing redox equations Step 1 Write an unbalanced equation Step 2 Separate the process into half reactions a Assign oxidation numbers for each atom b Identify and write
We can use the half reaction method to balance the equations of redox reactions occurring in aqueous solution In this method a redox equation is separated into two half reactions one Aug 14 2020 nbsp 0183 32 To balance a redox equation using the oxidation state method we conceptually separate the overall reaction into two parts an oxidation in which the atoms of one element
More picture related to Balancing Redox Equations

Balancing Redox Equations Worksheet Answers
http://www.worksheeto.com/postpic/2010/06/balancing-redox-reactions-worksheet-answers_236710.png

Balancing Redox Equations Change In Oxidation Method
https://s3.studylib.net/store/data/008287150_1-7905a2699304d856346da2c5bef2e97b-768x994.png
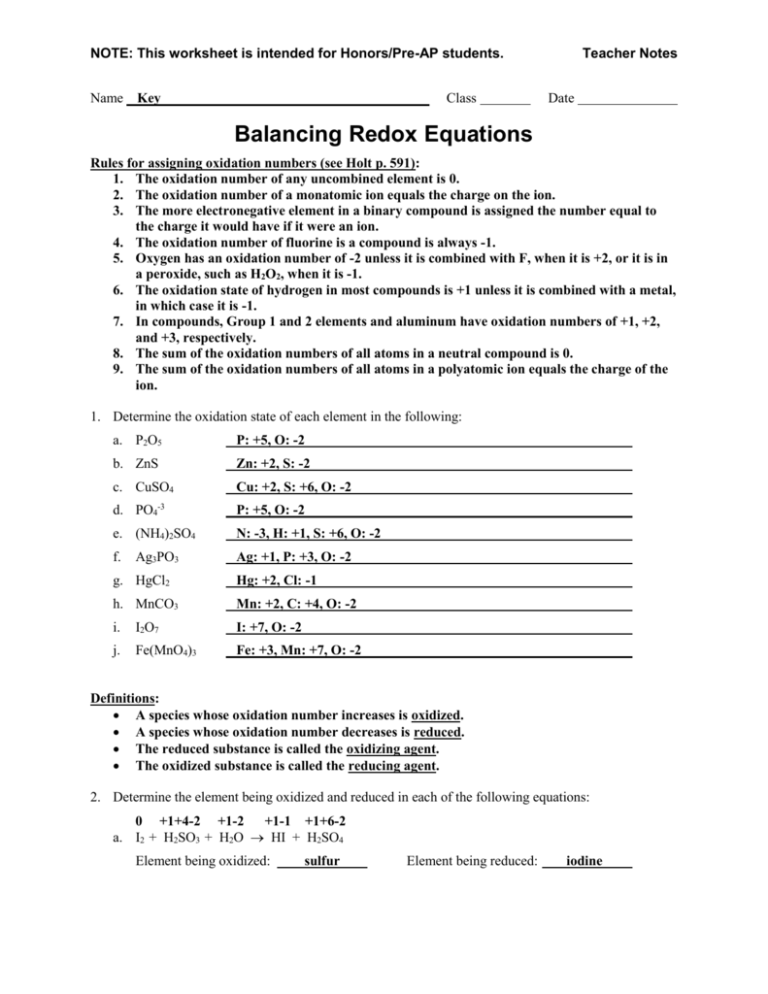
Worksheet 3 Worksheet Balancing Redox Equations
https://s3.studylib.net/store/data/007393601_1-8dcdc49a4293ea51fa2dfef3875bd687-768x994.png
There are different methods and several steps to balancing oxidation reduction equations also known as redox reactions These include Step 1 Write the unbalanced equation showing the 1 Electrons NEVER appear in a correct final answer In order to get the electrons in each half reaction equal one or both of the balanced half reactions will be multiplied by a factor 2
[desc-10] [desc-11]

Solved Practice Balancing Redox Equations 1 HNO3 aq Chegg
https://media.cheggcdn.com/media/145/1450b10b-8f81-4623-9c4f-490bb951b4ec/phpJblawT.png
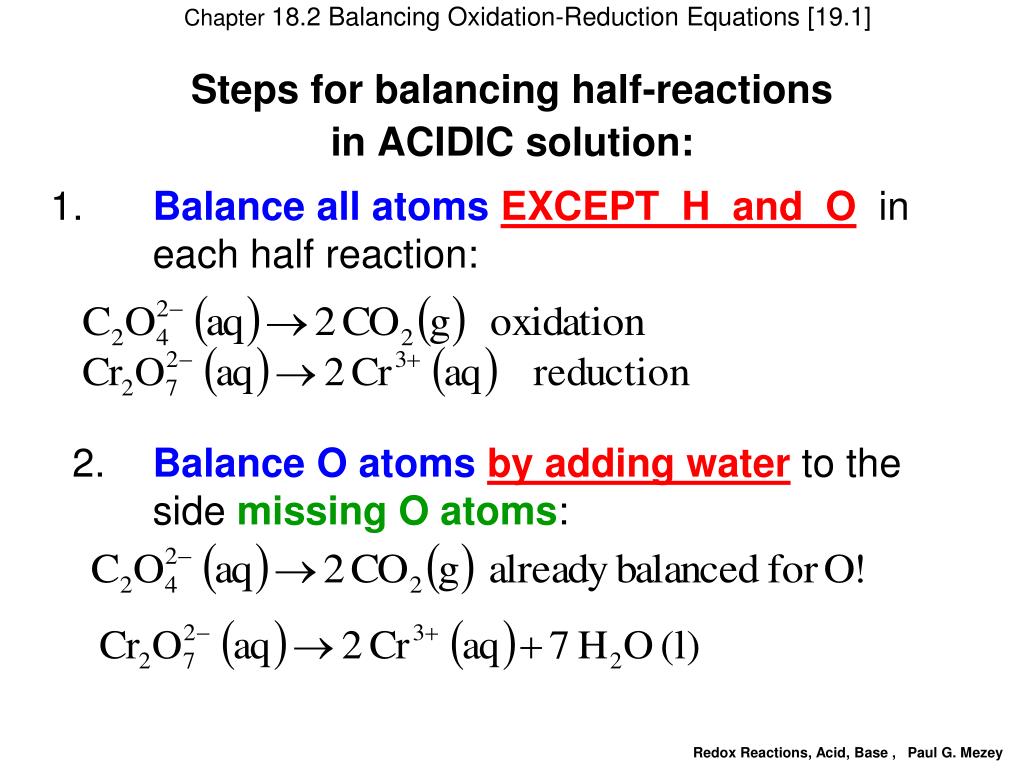
PPT Chapter 18 2 Balancing Redox Equations Redox Reactions
https://image3.slideserve.com/6127669/steps-for-balancing-half-reactions-in-acidic-solution-l.jpg
Balancing Redox Equations - [desc-13]