Balance Chemical Equations Jan 22 2025 nbsp 0183 32 To balance a chemical equation first write out your given formula with the reactants on the left of the arrow and the products on the right For example your equation should look something like quot H2 O2 H2O quot
Mar 14 2023 nbsp 0183 32 The Key to Balancing Chemical Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above Use uppercase for the first character in the element and lowercase for the second character Examples Fe Au Co Br C O N F Ionic charges are not yet supported and will be ignored
Balance Chemical Equations

Balance Chemical Equations
https://sciencenotes.org/wp-content/uploads/2015/01/balanceequations4-791x1024.png
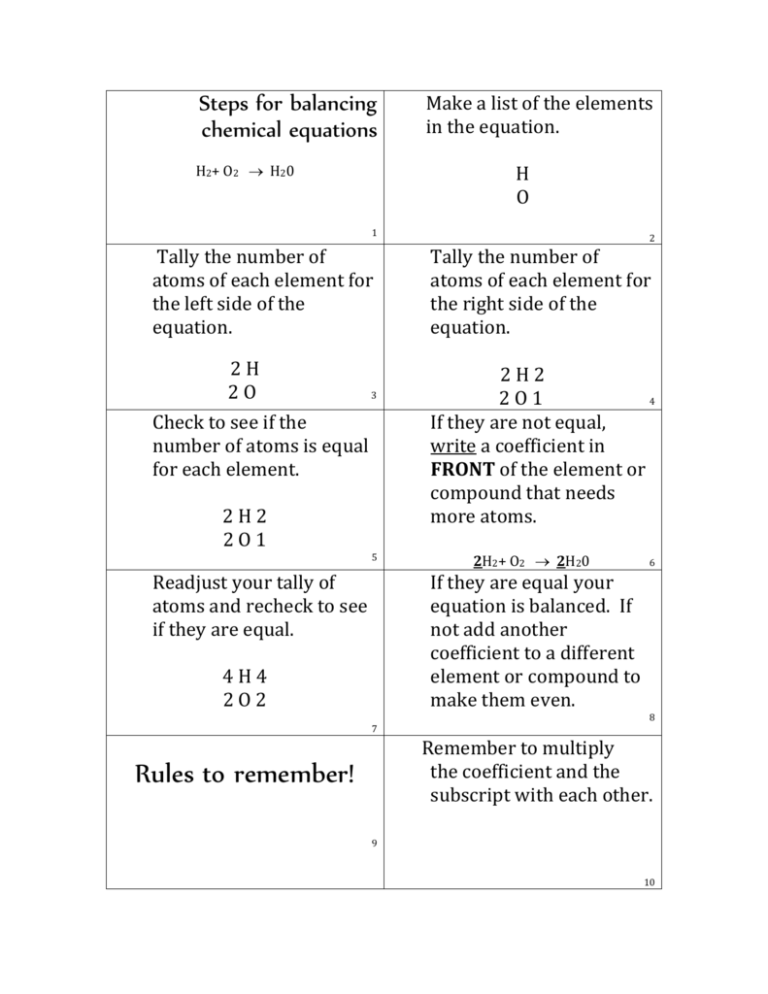
Basic Steps To Balance Chemical Equations Tessshebaylo
https://s3.studylib.net/store/data/006967896_1-a74c12e23c1c947d80a7b8b6ed37c209-768x994.png
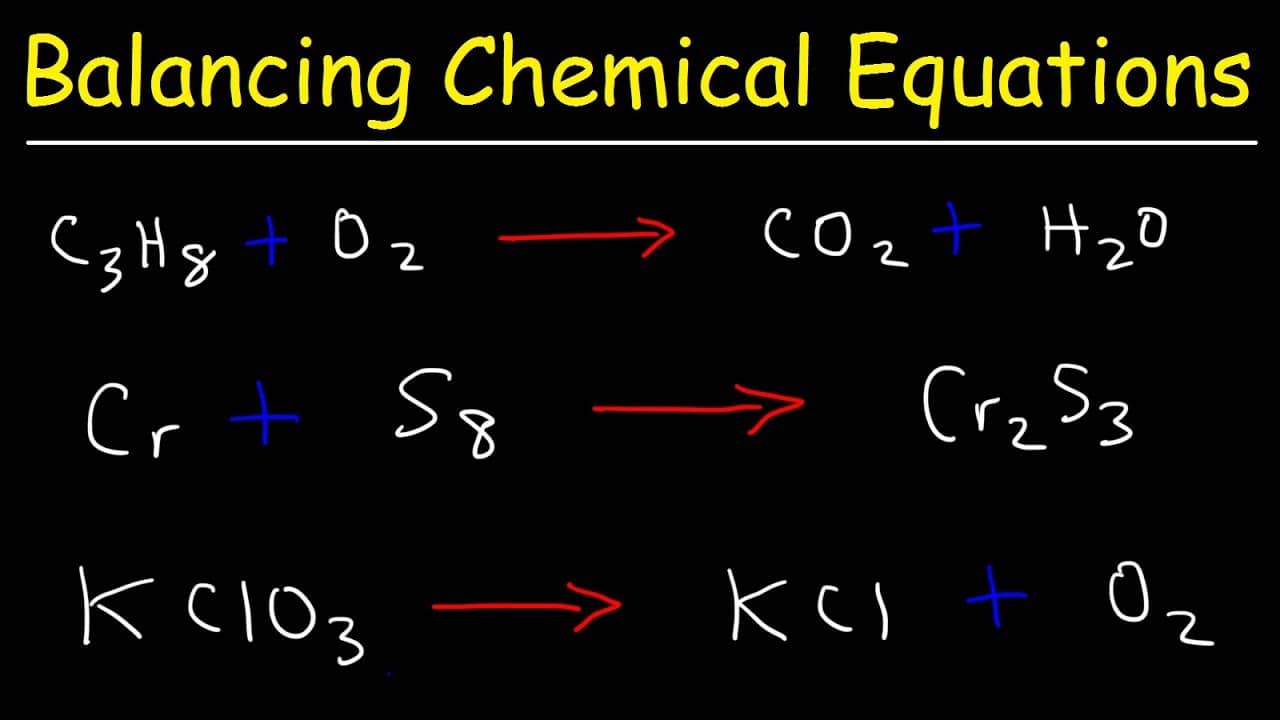
How To Balance Chemical Equations Best Examples Get Education Bee
https://geteducationbee.com/wp-content/uploads/2020/11/How-to-Balance-Chemical-Equations.jpg
Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions i e the same number of atoms of each element must exist on the reactant side and the product side of the equation This interactive tool is designed to help you practice balancing chemical equations with ease Simply input coefficients for each compound in the equation then click the Check Balance button to see if your equation is balanced If there are any discrepancies you ll receive instant feedback on what s off and can adjust accordingly
Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the Aug 23 2022 nbsp 0183 32 Balancing chemical equations involve the addition of stoichiometric coefficients to the reactants and products This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions i e the same number of atoms of each element must exist on the reactant side and the product side of the equation
More picture related to Balance Chemical Equations

Balance Chemical Equations Easy Worksheet Mastering The Art Of
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-18.jpg

How To Balance Chemical Equations Vrogue co
https://i.ytimg.com/vi/vDMTt0VZ_DQ/maxresdefault.jpg

Balance Chemical Equations Practice
https://1217986664s505.typepad.com/files/equationsws.jpg
Sep 30 2024 nbsp 0183 32 Add Coefficients To Balance Mass in a Chemical Equation When balancing chemical equations you never change subscripts You add coefficients Coefficients are whole number multipliers If for example you write 2 H 2 O that means you have 2 times the number of atoms in each water molecule which would be 4 hydrogen atoms and 2 oxygen atoms Using these chemical equations with balanced atoms leads to chemical reactions that produce the desired product That is why this balancing chemical equations calculator takes a couple of clicks in displaying the exact amount of each reactant and product to
[desc-10] [desc-11]
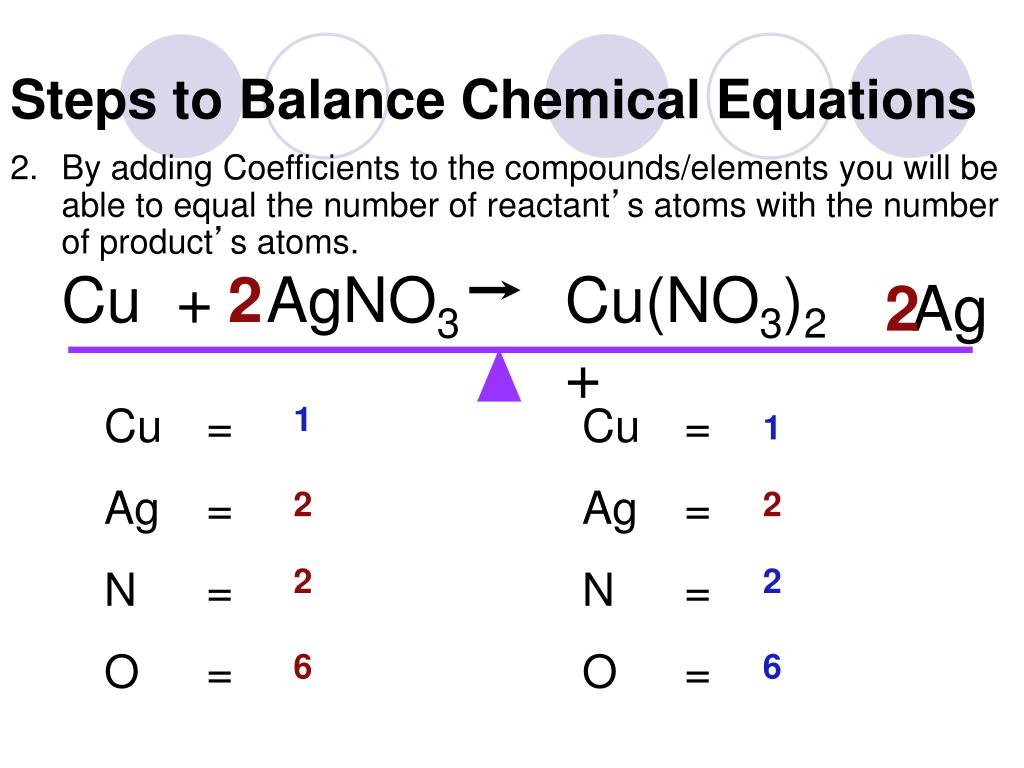
PPT How To Balance Chemical Equations PowerPoint Presentation Free
https://image2.slideserve.com/3850828/steps-to-balance-chemical-equations1-l.jpg

Easy Steps To Balance Chemical Equations Sale Online
https://chemsimplified.com/wp-content/uploads/2018/02/HowToBalanceChemicalEquations_Algebraic.jpg
Balance Chemical Equations - [desc-13]