Average Atomic Mass Calculation Example Average atomic masses listed by IUPAC are based on a study of experimental results Bromine has two isotopes 79 Br and 81 Br whose masses 78 9183 and 80 9163 amu and abundances 50 69 and 49 31 were determined in earlier experiments
Sep 22 2023 nbsp 0183 32 The average atomic mass tells you the relationship between mass and number of atoms in a typical sample of the element This is useful in chemistry laboratories because it is almost impossible to count the number of atoms directly but easy to measure mass Jan 18 2024 nbsp 0183 32 The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural abundance
Average Atomic Mass Calculation Example
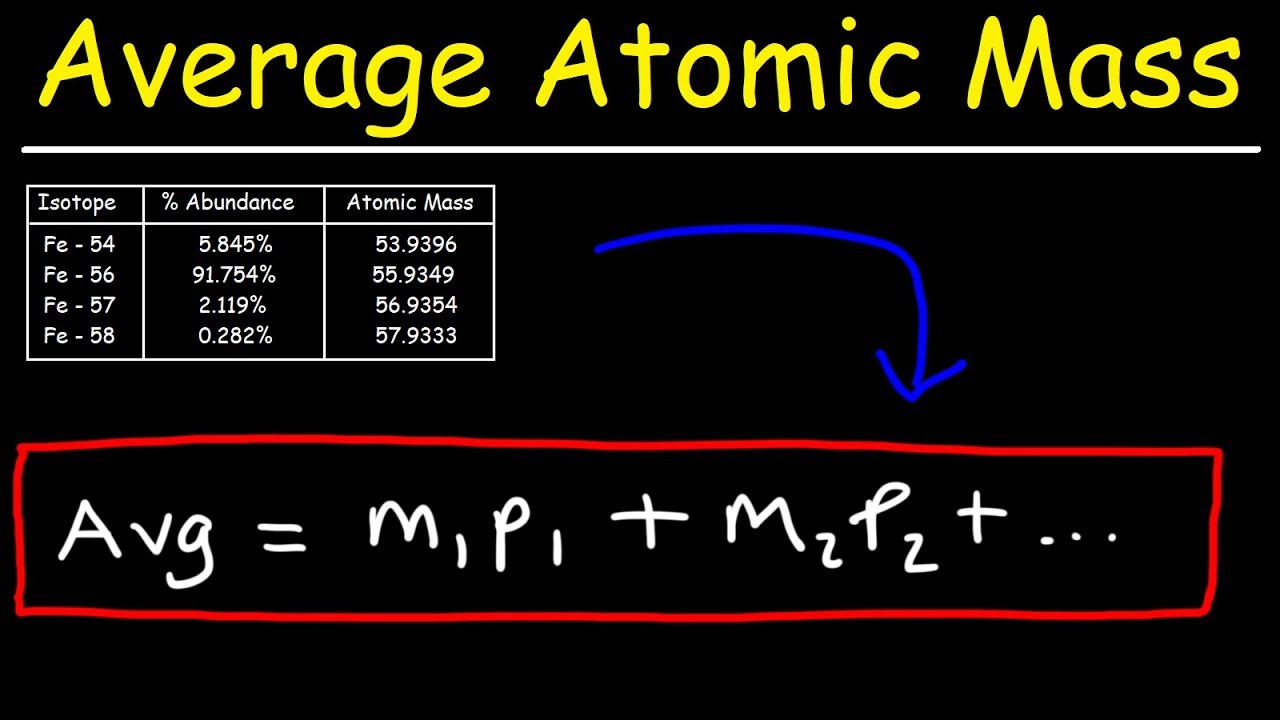
Average Atomic Mass Calculation Example
https://i.ytimg.com/vi/JT18bDAadQ0/maxresdefault.jpg

Simple Average Atomic Mass Calculation YouTube
https://i.ytimg.com/vi/hlUYxzexqSQ/maxresdefault.jpg

Calculating Average Atomic Mass Worksheet Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/190b01343490a8257c1a5d2a56d9a2be/thumb_1200_1553.png
Jul 18 2022 nbsp 0183 32 Using the masses of the different isotopes and how abundant each isotope is we can find the average mass of the atoms of an element The atomic mass of an element is the weighted average mass of the atoms in a naturally occurring sample of the element Atomic mass is typically reported in atomic mass units Try this average atomic mass calculator to determine the weighted mean of the masses of all isotopes of an element The calculator would weigh these masses based on their natural abundance yielding an average atomic mass of approximately 12 0107 amu for carbon
The average atomic mass is the average mass of all the isotopes that compose that element weighted based on the natural abundance of each isotope So how do we calculate the average atomic mass Let s see this by doing an example on chlorine Naturally occurring chlorine consists of 75 77 chlorine 35 atoms with an atomic mass of 34 97 amu Sep 19 2017 nbsp 0183 32 This example will show how to find the average atomic mass of an element when given the natural abundance of each of the element s isotopes Magnesium Mg element 12 has three natural isotopes Mg 24 Mg 25 and Mg 26
More picture related to Average Atomic Mass Calculation Example

How To Calculate The Atomic Mass Of An Isotope Average Atomic Mass
https://www.wikihow.com/images/e/eb/Find-Average-Atomic-Mass-Step-8.jpg
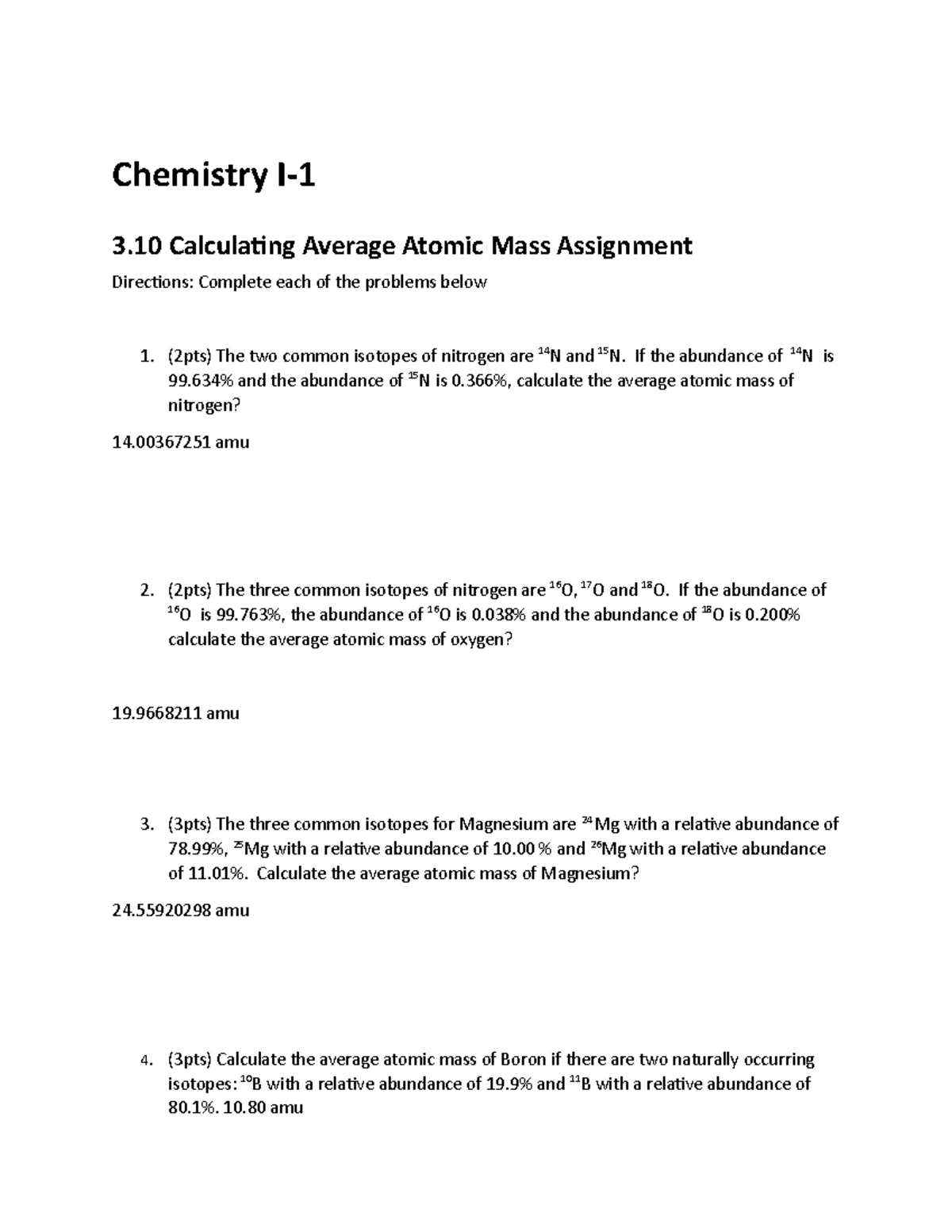
3 10 Average Atomic Mass Assignment Chemistry I 3 Calculating
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/529a6c6a023dc7cbc59efe768d694ffa/thumb_1200_1553.png
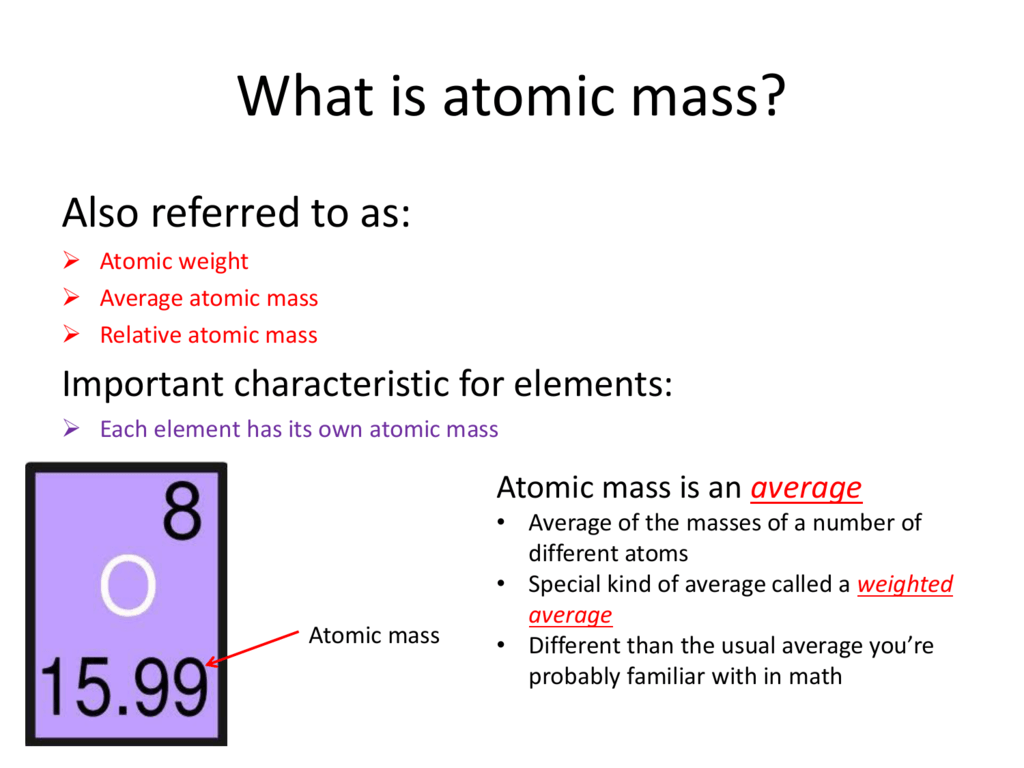
What Is Atomic Mass
https://s3.studylib.net/store/data/009081217_1-f4725e7d33e4609ef501d684c03ae8c6.png
The average atomic mass is the weighted average of all these isotopes based on their natural abundance An average atomic mass calculator helps to compute this weighted average making it easy for students and scientists to understand the mass of an element more accurately First we define what an average atomic mass is and then show steps to calculate it We also explain why the weighted average gives a more accurate value than the normal average by considering all isotopic elements of an element
The average atomic mass is calculated as the weighted average of the atomic masses of the naturally occurring isotopes of the element Example Calculating the Average Atomic Mass of Carbon Carbon has two stable isotopes carbon 12 C 12 and carbon 13 C 13 Aug 1 2024 nbsp 0183 32 Example Find the atomic mass of an isotope of carbon that has 7 neutrons You can see from the periodic table that carbon has an atomic number of 6 which is its number of protons The atomic mass of the atom is the mass of the protons plus the mass of the neutrons 6
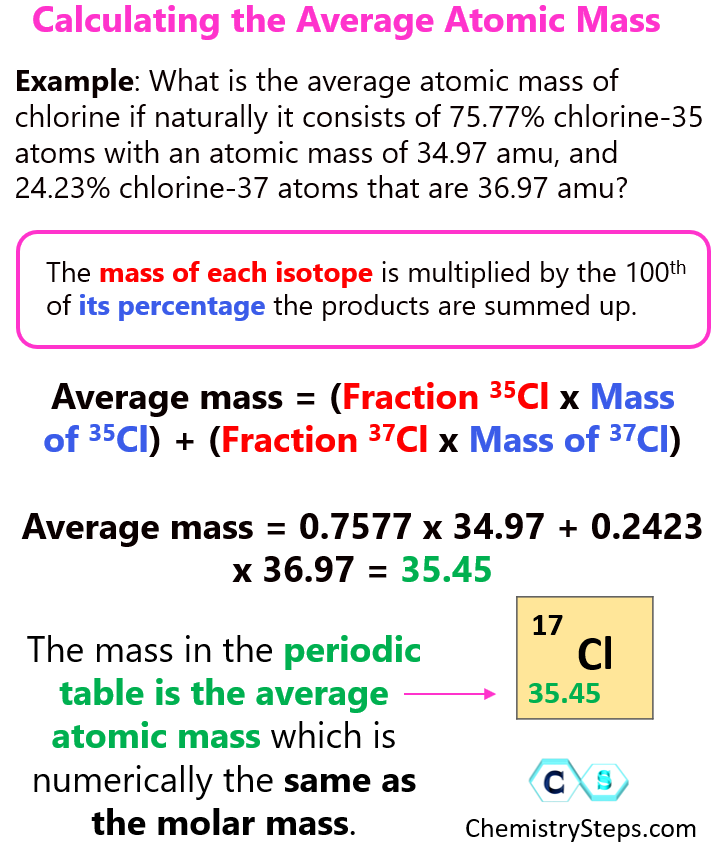
How To Calculate The Average Atomic Mass Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2023/01/How-To-Calculate-The-Average-Atomic-Mass.png
Isotopes
http://www.sliderbase.com/images/referats/1140b/(23).PNG
Average Atomic Mass Calculation Example - Dec 20 2021 nbsp 0183 32 Atomic masses are calculated from weighted averages of all that element s isotopes based on their abundance Typically homework problems will provide you with a sample of an element with a list of all isotopes their mass and their percent abundance To solve these problems follow these two steps
.PNG)