A Helium Atom With 2 Protons 2 Neutrons Helium 3 is a light stable isotope of helium with two protons and one neutron the most common isotope helium 4 having two protons and two neutrons in contrast Other than protium ordinary hydrogen helium 3 is the only stable isotope of any element with more protons than neutrons
The number of neutrons is 2 and the helium atom always has 2 protons check the atomic number 2 of helium Thus the mass number is the sum of protons and neutrons in the nucleus 2 2 4 A helium atom is an atom of the chemical element helium Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons depending on the isotope held together by the strong force
A Helium Atom With 2 Protons 2 Neutrons

A Helium Atom With 2 Protons 2 Neutrons
https://i0.wp.com/valenceelectrons.com/wp-content/uploads/2022/06/Helium-protons-neutrons-electrons.jpg
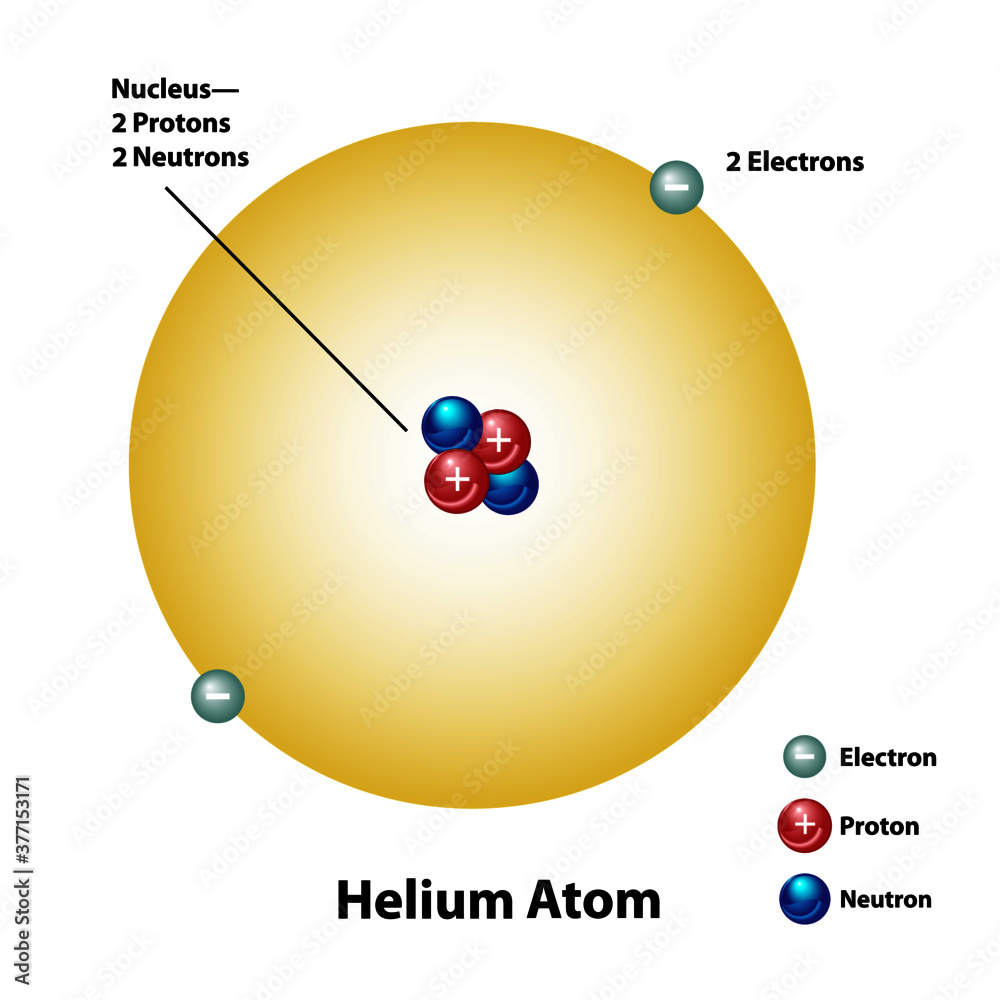
Helium Molecular Element Diagram Showing Mass Protons Electrons
https://as2.ftcdn.net/v2/jpg/03/77/15/31/1000_F_377153171_RPztLk6nlIqTSqPTqXvX3BJqKwB5YTOp.jpg
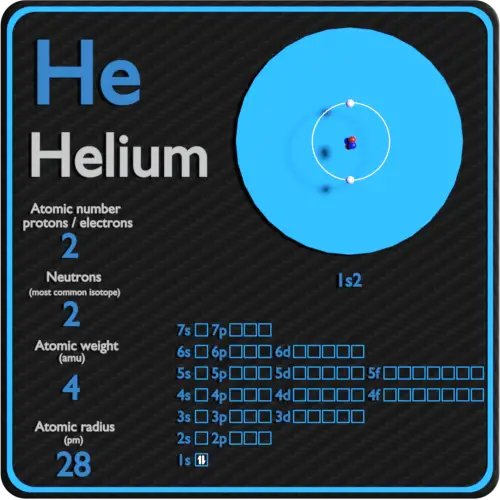
Helium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/09/Helium-protons-neutrons-electrons-configuration.png
Dec 3 2019 nbsp 0183 32 The isotope containing 2 protons and 2 neutrons is called helium 4 There are nine isotopes of helium but only helium 3 and helium 4 are stable In the atmosphere there is one atom of helium 3 for every million helium 4 atoms Aug 22 2024 nbsp 0183 32 We know that the atomic number of helium is 2 and the atomic mass number is about 4 Neutron 4 2 2 Therefore a helium atom has two neutrons Based on the atomic number mass number and neutron number of the element three things can be considered These are isotope isobar and isotone
Element Helium He Group 18 Atomic Number 2 s block Mass 4 003 Sources facts uses scarcity SRI podcasts alchemical symbols videos and images Helium is the 2nd element in the periodic table and has a symbol of He and atomic number of 2 It has an atomic weight of 4 00260 and a mass number of 4 Helium has two protons and two neutrons in its nucleus and two electrons in one shell
More picture related to A Helium Atom With 2 Protons 2 Neutrons
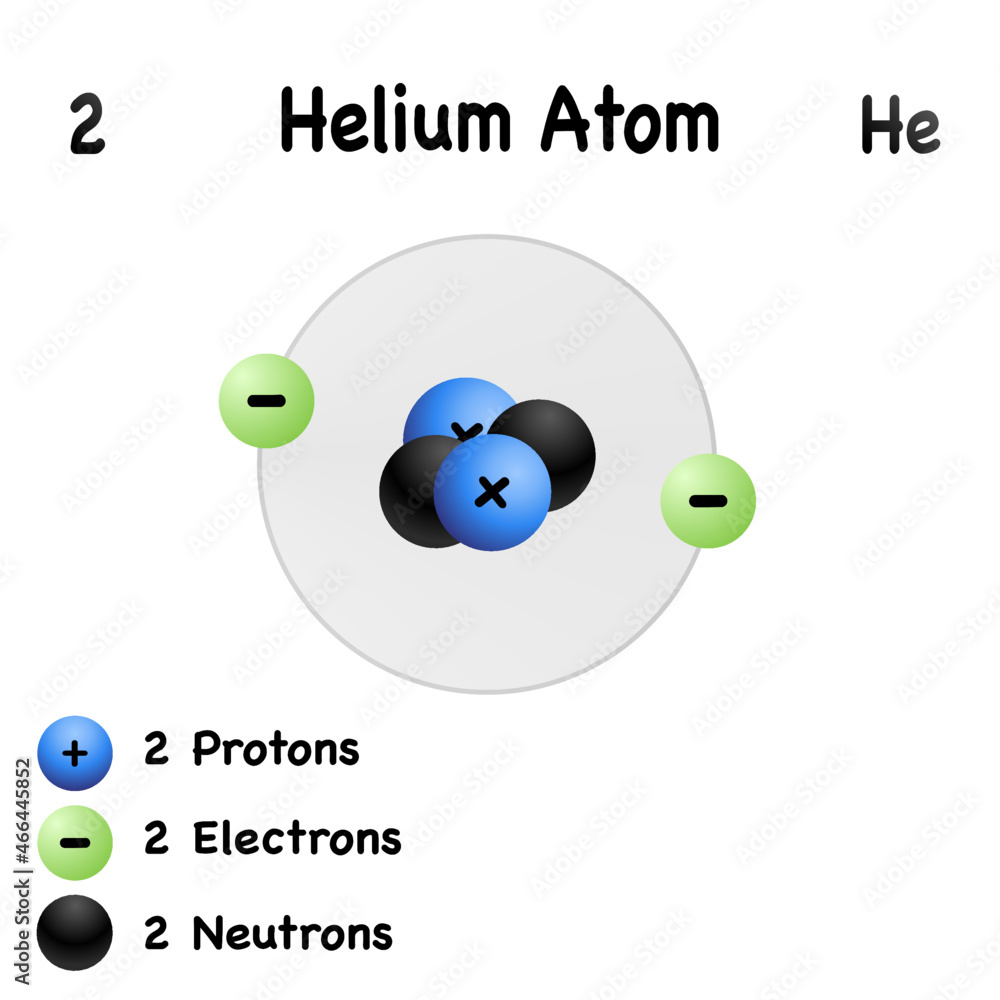
Helium Element With Symbol He And Atomic Number 2 isolated Molecular
https://as1.ftcdn.net/v2/jpg/04/66/44/58/1000_F_466445852_MejuX8oUAeZrqTDbxiMD3c0QRr9gXvBm.jpg
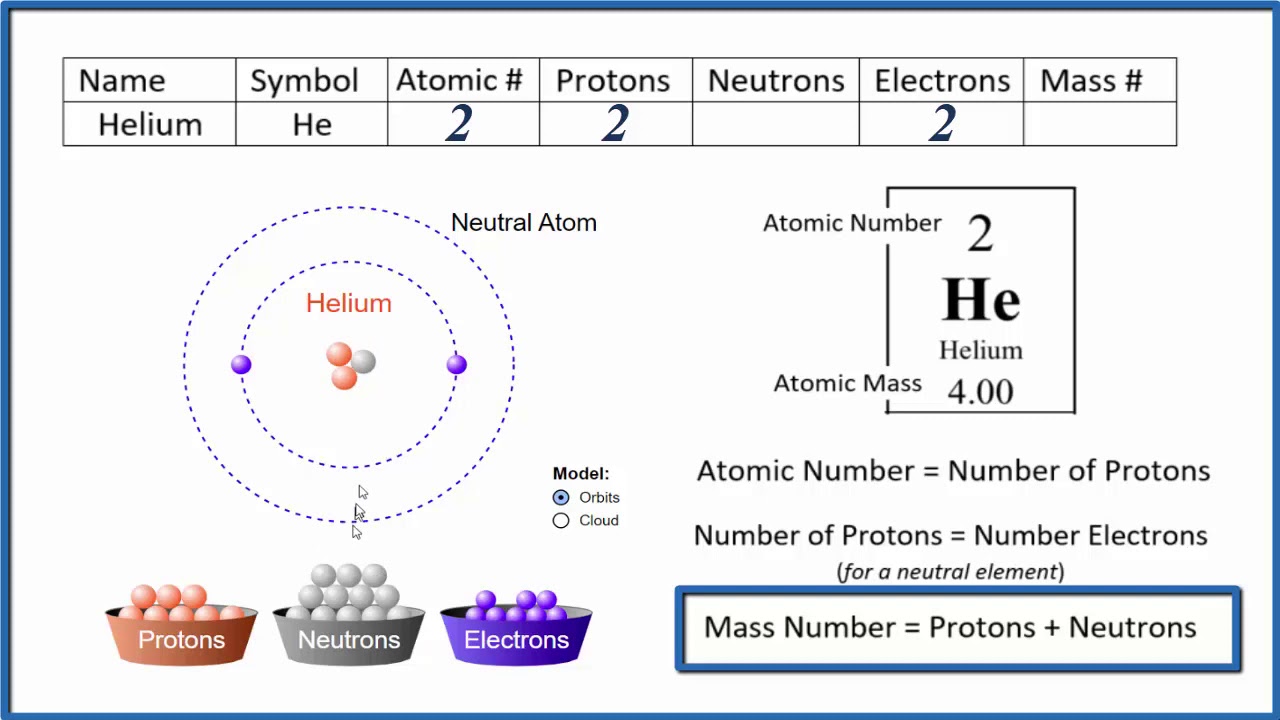
How To Find The Number Of Protons Electrons Neutrons For Helium He
https://i.ytimg.com/vi/tgPo9sUhcs4/maxresdefault.jpg

2 1 Elements And Atoms The Building Blocks Of Matter Douglas College
https://pressbooks.bccampus.ca/dcbiol12031209/wp-content/uploads/sites/150/2017/08/202_Two_Models_of_Atomic_Structure-4.jpg
May 18 2015 nbsp 0183 32 The natural isotopes of helium are helium 3 which has two protons and one neutron and helium 4 which has two protons and two neutrons Both isotopes are stable He 3 accounts for 1 37 x 10 4 of helium found in the atmosphere Atomic Number Protons Electrons and Neutrons in Helium Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z
Jan 27 2020 nbsp 0183 32 The atomic number of helium is 2 meaning each atom of helium has two protons The most abundant isotope of the element has 2 neutrons It is energetically favorable for each helium atom to have 2 electrons which gives it a stable electron shell Jul 3 2007 nbsp 0183 32 Helium is the second most abundant element comprising roughly one quarter of the mass of the Universe This animation zooms into a standard helium atom showing its protons green neutrons white and electrons blue

Helium Atom Plugon
https://storage.googleapis.com/plugbucket/pub/up/9/9c3/9c36015803c448cf801f250b9055d574/img1.jpg
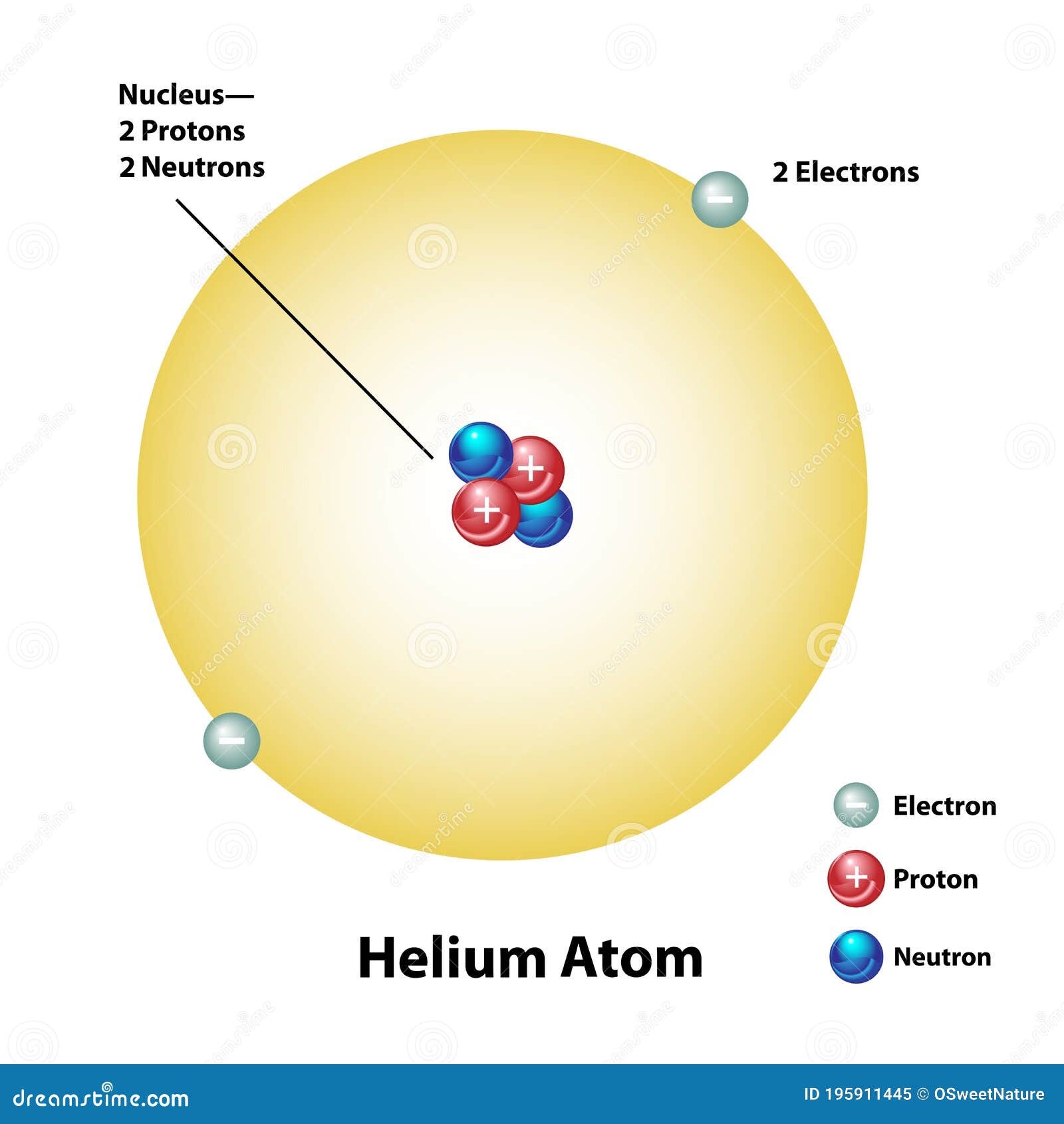
Helium Atom With Nucleus And Electron Shell Stock Vector Illustration
https://thumbs.dreamstime.com/z/heliumatom-195911445.jpg
A Helium Atom With 2 Protons 2 Neutrons - Helium is the 2nd element in the periodic table and has a symbol of He and atomic number of 2 It has an atomic weight of 4 00260 and a mass number of 4 Helium has two protons and two neutrons in its nucleus and two electrons in one shell