Why Are Binary Ionic Compounds Easily Formed Jul 29 2022 nbsp 0183 32 Magnesium and nitrogen react to form an ionic compound Predict which forms an anion which forms a cation and the charges of each ion Write the symbol for each ion and name them
Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl NaCl is a binary ionic compound Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We
Why Are Binary Ionic Compounds Easily Formed

Why Are Binary Ionic Compounds Easily Formed
https://www.compoundworksheets.com/wp-content/uploads/2023/03/naming-binary-ionic-compounds-worksheet-education-template-5.jpg

What Are Binary Ionic Compounds Formation And Naming YouTube
https://i.ytimg.com/vi/mdD60FeZpwQ/maxresdefault.jpg

Naming Binary Ionic Compounds YouTube
https://i.ytimg.com/vi/CTvvJRGMdxY/maxresdefault.jpg
Ionic bonds are formed between cations and anions A cation is formed when a metal ion loses a valence electron while an anion is formed when a non metal gains a valence electron They both achieve a more stable electronic Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We can think about the formation of such compounds
The Formation of Ionic Compounds Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We can think Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We
More picture related to Why Are Binary Ionic Compounds Easily Formed
Solved Write The Empirical Formula Of At Least Four Binary Ionic
https://www.coursehero.com/qa/attachment/15815424/
Properties Of Ionic Compounds And Naming formula Writing For Simple
https://wordwallscreens.azureedge.net/800/aaf054e9f82a4a4dbdaaa7bdd8e6b593_0
Solved NOMENCLATURE AND FORMULA WRITING BINARY IONIC COMPOUNDS
https://www.coursehero.com/qa/attachment/35517373/
Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We Remember that ions are formed only when electrons move from one atom to another a proton never moves from one atom to another Compounds formed from positive and negative ions
What are Ionic Compounds Ionic compounds consist of positive ions called cations and negative ions called anions hence ionic compounds often consist of a metal and non metal Learn more about structure properties amp examples of The simplest ionic compounds are binary ionic compounds or those that only contain two atoms one acting as the cation and one acting as the anion Thus we will focus on the formation of

Ionic Bonding Wikipedia
https://upload.wikimedia.org/wikipedia/commons/0/0d/Ionic_Bonds.png
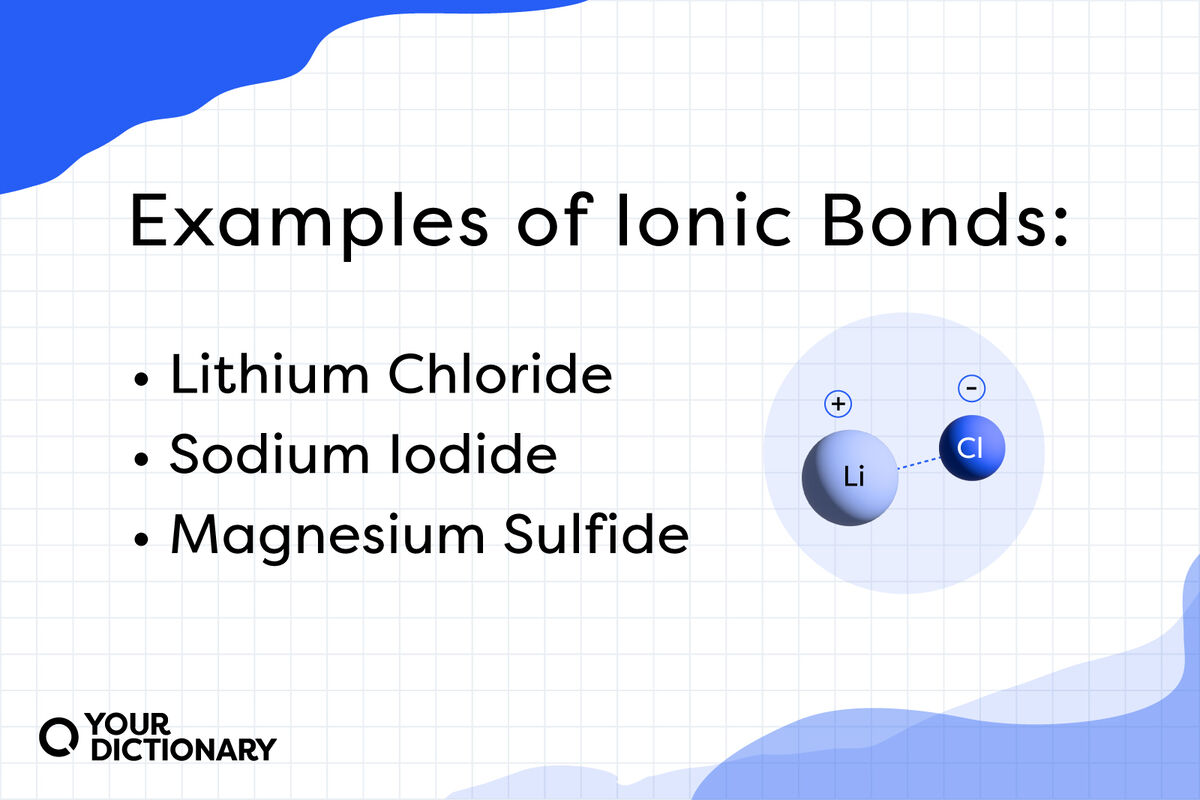
Ionic Compound List
https://assets.ltkcontent.com/images/1664604/Ionic-Bond-Examples-22_27c5571306.jpg
Why Are Binary Ionic Compounds Easily Formed - Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We