Which Molecule Shape Is Always Polar Aug 10 2022 nbsp 0183 32 A polar molecule is a molecule in which one end of the molecule is slightly positive while the other end is slightly negative A diatomic molecule that consists of a polar covalent bond such as ce HF is a polar molecule
Dec 13 2019 nbsp 0183 32 Here s a look at each confirmed molecular geometry T shaped A This shape features a central atom with two bonds oriented in a plane and one bond extending perpendicularly The presence of fluorine will create a resultant dipole making this shape polar Nov 14 2024 nbsp 0183 32 Molecules with asymmetrical shapes such as those with lone pairs on the central atom are always polar This includes shapes like bent trigonal pyramidal and seesaw
Which Molecule Shape Is Always Polar

Which Molecule Shape Is Always Polar
https://physvids.com/wp-content/uploads/2022/10/Atoms-PolarBond-1024x576.jpg

Which Of The Following Molecules Is are Polar For Each Po Quizlet
https://slader-solution-uploads.s3.amazonaws.com/e9890243-8447-42bc-98a3-ebd53dd5cad7-1638450712791130.png
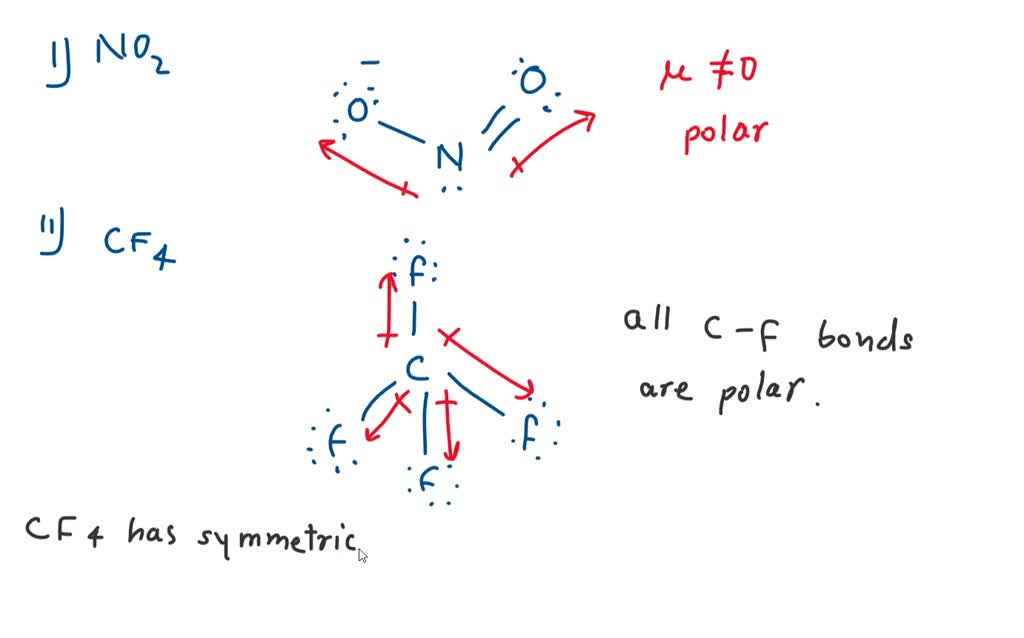
SOLVED Which Molecule Has Polar Bonds But Is Non polar A ClF3 B H20
https://cdn.numerade.com/ask_previews/be7af51d-9c5e-44a3-8e36-a0e92189b959_large.jpg
Feb 15 2023 nbsp 0183 32 Molecular shapes that are always nonpolar include those with spherical symmetry such as the tetrahedral shape In these cases the electrons are evenly distributed around the central atom resulting in a nonpolar molecule Square pyramid shaped molecules on the other hand are always polar What 5 molecular geometries are always polar 1 EG tetrahedral MG trigonal planar 2 EG tetrahedral MG bent 3 EG Trig Bipy MG seesaw 4 EG trig bipy MG T shape 5 EG Octahedral MG square pyramidal Study with Quizlet and memorize flashcards containing terms like EDG Linear EDG Trigonal Planar EDG Tetrahedral and more
May 7 2020 nbsp 0183 32 A polar molecule has an asymmetric shape lone electron pair or central atom bonded to other atoms with different electronegativity values Usually a polar molecule contains ionic or polar covalent bonds The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms The molecular polarity can be established by determining the vector sum of all bond dipoles
More picture related to Which Molecule Shape Is Always Polar

Molecular Polarity Table Molecular Geometry Molecular Shapes Molecular
https://i.pinimg.com/736x/4c/72/ed/4c72ed42a8b007fe71eb9201118abcc6--math-activities-chemistry.jpg

VSEPR Polarity And Bonds YouTube
https://i.ytimg.com/vi/40mG2rQlLpk/maxresdefault.jpg

Polarity Definition Examples Britannica
https://cdn.britannica.com/04/96904-050-B709B54E/atoms-bond-bonds-electrons-hydrogen-oxygen-atom.jpg
Due to the non symmetrical shape of the molecule bent the molecule itself is polar it has a delta and delta side Carbon tetrachloride has four polar covalent bonds Jan 15 2024 nbsp 0183 32 If there is only one bond in the molecule the bond polarity determines the molecular polarity Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule A diatomic molecule that consists of a polar covalent bond such as HF is a polar molecule where one end of the molecule is slightly positive while the other end is slightly
A molecule that contains polar bonds might not have any overall polarity depending upon its shape The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap If these centers lie at the same point in space then the molecule has no overall polarity and The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms The molecular polarity can be established by determining the vector sum of all bond dipoles
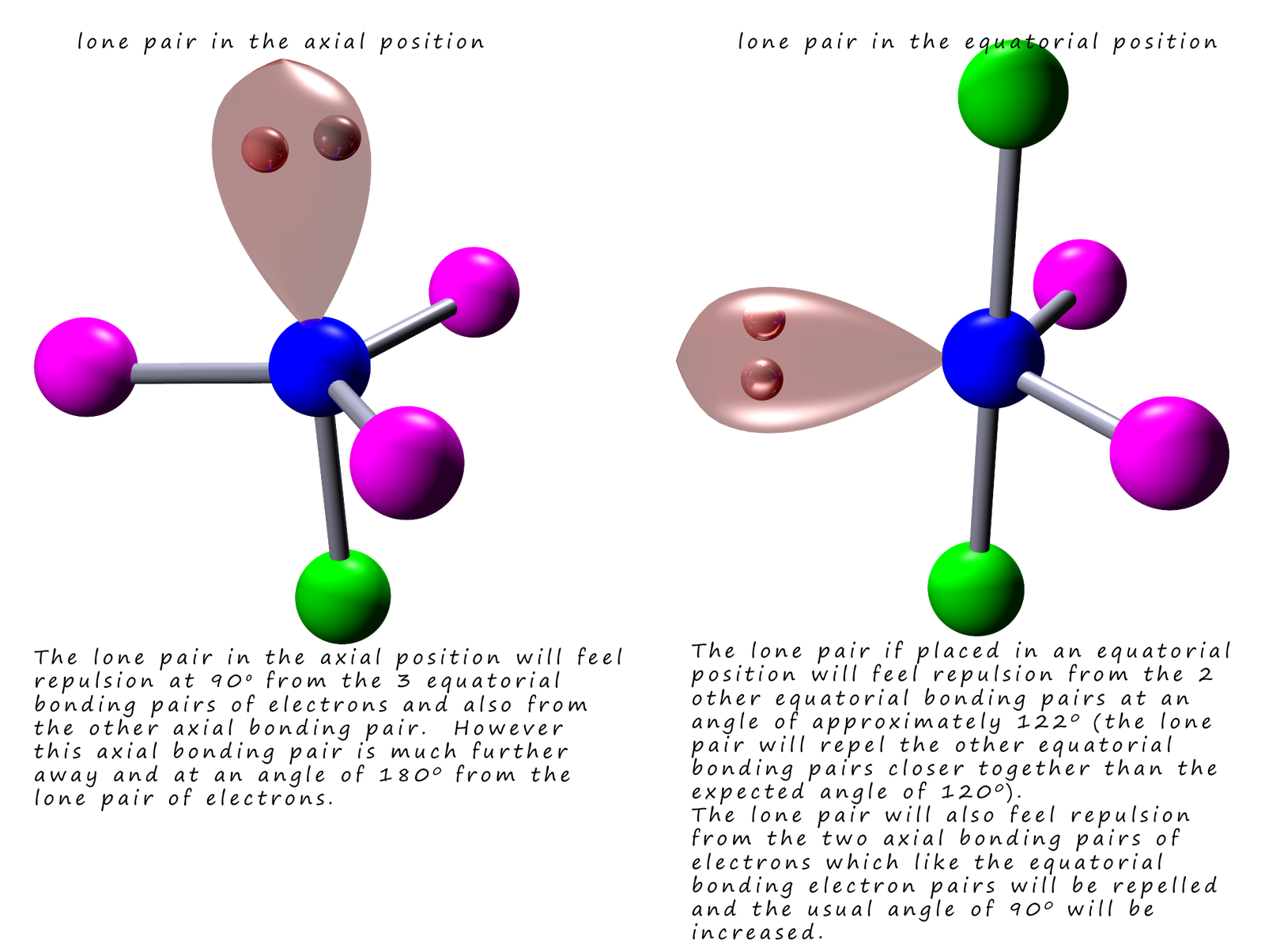
Tbp Molecules And Lone Pairs
https://www.science-revision.co.uk/images/shapes tbp axial and equatorial lone pairs.png
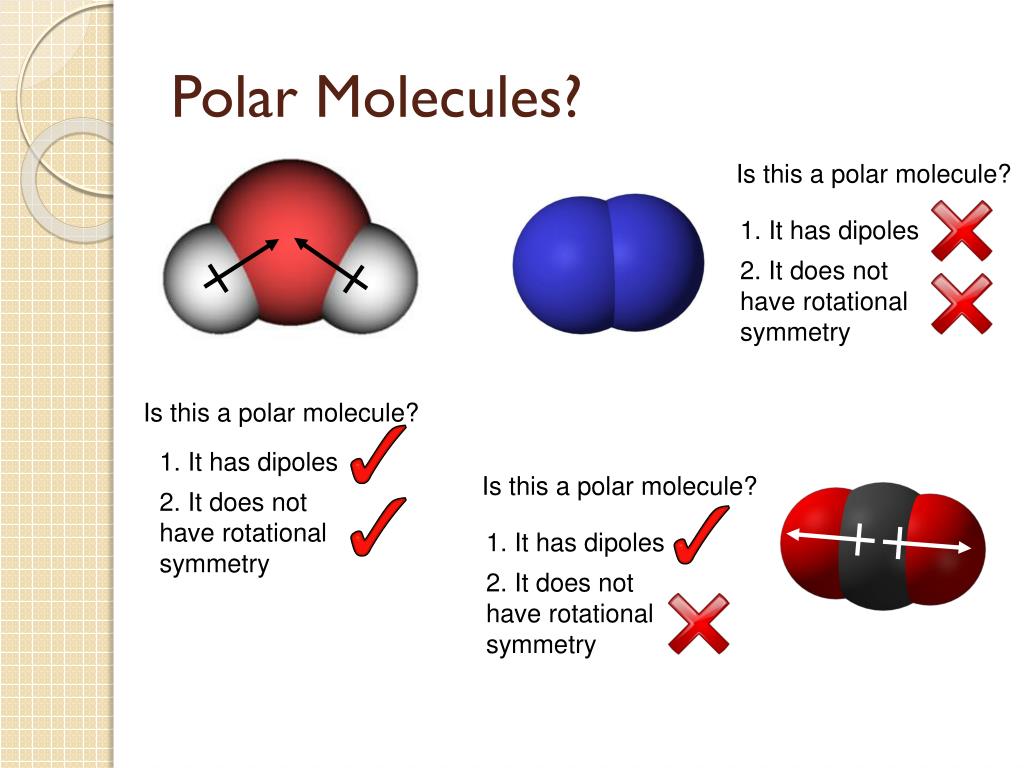
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint
https://image2.slideserve.com/4261659/polar-molecules1-l.jpg
Which Molecule Shape Is Always Polar - Feb 15 2023 nbsp 0183 32 Molecular shapes that are always nonpolar include those with spherical symmetry such as the tetrahedral shape In these cases the electrons are evenly distributed around the central atom resulting in a nonpolar molecule Square pyramid shaped molecules on the other hand are always polar