Which Element Has 3 Electrons 3 Protons And 4 Neutrons Solution a Atomic number Number of protons 3 b Mass number Number of protons number of neutrons 3 4 7 c Electronic configuration of the atom is 2 1 K has filled with 2
May 27 2023 nbsp 0183 32 Here is the Elements protons neutrons and electrons list Atomic no Elements Protons Neutrons and Electrons of Elements 1 Hydrogen Hydrogen has 1 proton 0 neutron and 1 electron 2 They are arranged to highlight similarities between different elements An atom has 3 protons 4 neutrons and 3 electrons Use the periodic table to determine which atom would have similar
Which Element Has 3 Electrons 3 Protons And 4 Neutrons

Which Element Has 3 Electrons 3 Protons And 4 Neutrons
https://www.wikihow.com/images/0/0f/Find-the-Number-of-Protons,-Neutrons,-and-Electrons-Step-5.jpg
Understanding Protons Electrons And Neutrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Electron Proton Neutron
https://d1avenlh0i1xmr.cloudfront.net/9608f5f5-e406-4832-bf83-648f0c2a0609/13.-aluminium-atom-teachoo-01.png
Jun 15 2024 nbsp 0183 32 Best Answer The only element with three protons is lithium The isotope lithium 6 has 3 neutrons and is the rarer of the two stable isotopes of lithium Most lithium is isotope Number of Electrons Atomic Number Number of Neutrons Atomic Mass Atomic Number In the below table we list the number of protons neutrons and electrons for each element in the
Sep 1 2024 nbsp 0183 32 The atomic number number at the top is the amount of protons and the amount of electrons So if an element has an atomic number of 5 you know that it has 5 protons and 5 electrons The atomic mass number at the Jul 29 2022 nbsp 0183 32 Describe the locations charges and masses of the three main subatomic particles Determine the number of protons and electrons in an atom Write and interpret symbols that
More picture related to Which Element Has 3 Electrons 3 Protons And 4 Neutrons
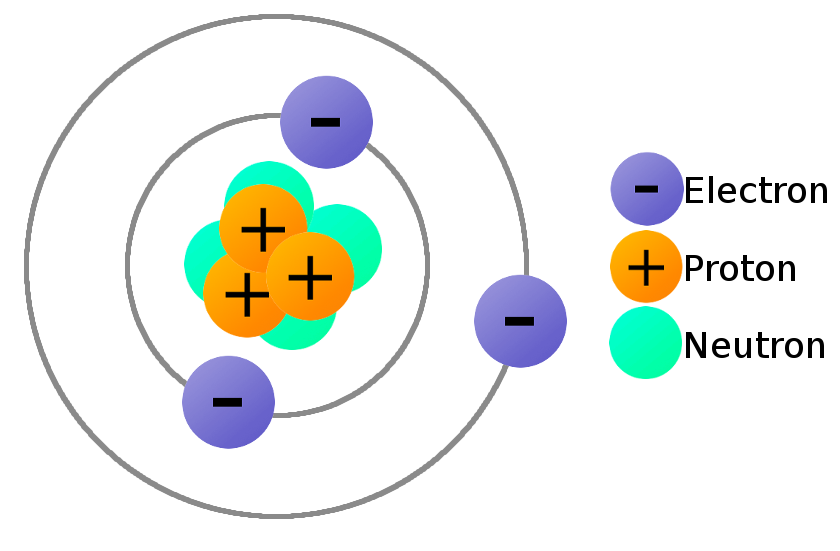
Do Cells Contain Atoms At Tony Myers Blog
https://dlnmh9ip6v2uc.cloudfront.net/assets/3/4/1/a/3/51a65d7bce395f156c000000.png

How To Find The Protons Neutrons And Electrons Of An Element On The
https://findstuffsonline.com/wp-content/uploads/2020/11/the-protons-neutrons-and-electrons-of-an-element-on-the-periodic-table-yooYnW8uhHk.jpg

Electrons Structure Properties Expii
https://d20khd7ddkh5ls.cloudfront.net/the_atom.jpeg
Today they agree that atoms have a positively charged nucleus made of protons and neutrons and negatively charged electrons that orbit the nucleus in shells From the periodic table we find that it has 29 protons The mass number 65 is the sum of the number of protons and neutrons Therefore we can subtract the number of protons from the
An element has 4 neutrons 3 electrons and 3 protons Which element does this describe Is it a neutral atom isotope or an ion What is the mass number What is the net charge Each element has a unique number of protons An element s atomic number is equal to the number of protons in the nuclei of any of its atoms The mass number of an atom is the sum of
Periodic Table Of Elements List With Protons Neutrons And Electrons
https://lh6.googleusercontent.com/proxy/rs2S-Rj8rlSVdk-V1vnR6XfXL1GxzwBipBXZfeum8ncIVsRoKqzZmpwPngddPtH1o4XHtcOhfhMXOVE71CwdXTDZjVqJmhWxqXLP4vXpWkg-pY9BNskkPmQ35GYRN2mYOihDWk6x9YhTygdPSIKiqYi0DJNUnnyuaO8TVkiuI1M6wHo=s0-d

Lithium s Atomic Structure YouTube
https://i.ytimg.com/vi/jmbHAa0JVlA/maxresdefault.jpg
Which Element Has 3 Electrons 3 Protons And 4 Neutrons - Jul 29 2022 nbsp 0183 32 Describe the locations charges and masses of the three main subatomic particles Determine the number of protons and electrons in an atom Write and interpret symbols that