Which Element Has 2 Protons And 2 Neutrons In Its Nucleus The element that has 2 protons and 2 neutrons is Helium He Atomic Structure of Helium The atomic structure of Helium is as follows Atomic number 2 Atomic mass 4 Number of protons 2 Number of neutrons 2 Number of electrons 2 The atomic number of an element is equal to the number of protons in its nucleus
Dec 3 2019 nbsp 0183 32 Helium is the element that is atomic number 2 on the periodic table Each helium atom has 2 protons in its atomic nucleus The atomic weight of the element is 4 0026 Helium does not readily form compounds so it is known in its pure form as a gas The number of neutrons is 2 and the helium atom always has 2 protons check the atomic number 2 of helium Thus the mass number is the sum of protons and neutrons in the nucleus 2 2 4
Which Element Has 2 Protons And 2 Neutrons In Its Nucleus
Which Element Has 2 Protons And 2 Neutrons In Its Nucleus
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Proton And Electron Periodic Table Periodic Table Printable
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/periodic-table-of-elements-list-with-protons-neutrons-and-electrons-scaled.jpg?resize=1536%2C1164&ssl=1
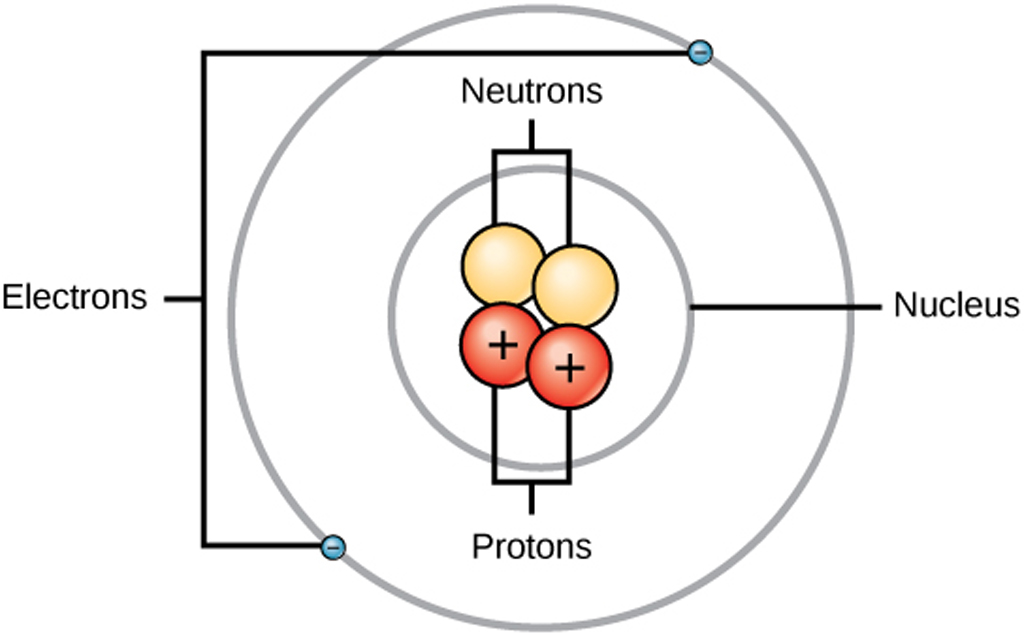
Structure Of An Atom Structure Use Of Electron Proton In Electronics
http://www.electronicsandyou.com/blog/wp-content/uploads/2015/07/atom-structure.jpg
Scientists distinguish between different elements by counting the number of protons in the nucleus Table 4 5 1 4 5 1 If an atom has only one proton we know that it s a hydrogen atom An atom with two protons is always a helium atom If scientists count four protons in an atom they know it s a beryllium atom Jul 29 2022 nbsp 0183 32 The atomic number of iodine 53 tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus Because the sum of the numbers of protons and neutrons equals the mass number 127 the number of
Jun 2 2019 nbsp 0183 32 The element of an atom with 2 protons is always helium If you are given the atomic weight of an atom you need to subtract the number of neutrons to get the number of protons Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight Today they agree that atoms have a positively charged nucleus made of protons and neutrons and negatively charged electrons that orbit the nucleus in shells
More picture related to Which Element Has 2 Protons And 2 Neutrons In Its Nucleus
7 Pics Periodic Table Of Elements List With Protons Neutrons And
https://alquilercastilloshinchables.info/wp-content/uploads/2020/06/95-PERIODIC-TABLE-ELEMENTS-PROTONS-NEUTRONS-ELECTRONS.jpg

Electron Proton Neutron
https://d1avenlh0i1xmr.cloudfront.net/9608f5f5-e406-4832-bf83-648f0c2a0609/13.-aluminium-atom-teachoo-01.png

Protons Neutrons And Subatomic Particles Pharmacy Gyan
https://pharmacygyan.com/wp-content/uploads/2021/09/protons.jpeg
Protons and neutrons are fermions with different values of the strong isospin quantum number so two protons and two neutrons can share the same space wave function since they are not identical quantum entities In this explainer we will learn how to work out the number of protons neutrons and electrons an atom of an isotope has from its chemical symbol The nucleus of an atom contains protons and neutrons All atoms of a given element have the same number of protons in their nucleus
Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells The numbers of subatomic particles in an atom can be calculated from its atomic number and mass Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells The numbers of subatomic particles in an atom can be calculated from its atomic number and mass
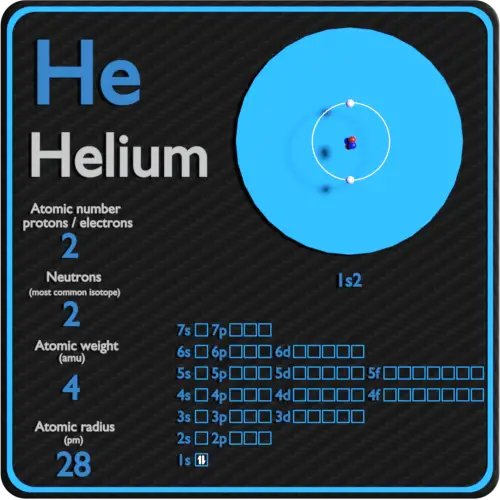
Helium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/09/Helium-protons-neutrons-electrons-configuration.png
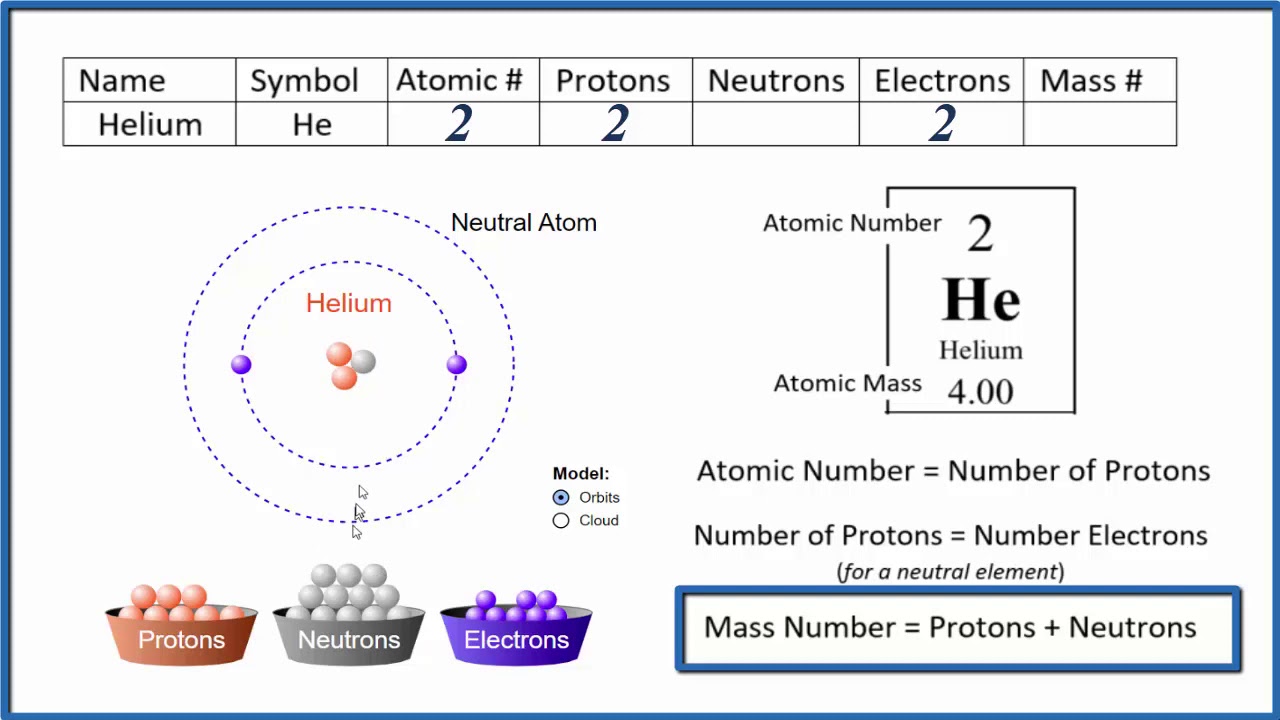
How To Find The Number Of Protons Electrons Neutrons For Helium He
https://i.ytimg.com/vi/tgPo9sUhcs4/maxresdefault.jpg
Which Element Has 2 Protons And 2 Neutrons In Its Nucleus - Jul 29 2022 nbsp 0183 32 The atomic number of iodine 53 tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus Because the sum of the numbers of protons and neutrons equals the mass number 127 the number of