What Molecular Shape Is Always Polar Feb 3 2025 nbsp 0183 32 When a molecule has a symmetrical shape such as linear or tetrahedral it is nonpolar However when a molecule has an asymmetrical shape like bent or trigonal
Nov 9 2023 nbsp 0183 32 The molecular shape that will always be polar is bent A bent molecule has a bent or V shape with a central atom and two surrounding atoms The bond dipoles do not cancel The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms The molecular polarity can be established by determining
What Molecular Shape Is Always Polar

What Molecular Shape Is Always Polar
https://i.ytimg.com/vi/sZVw8b8n_eU/maxresdefault.jpg

Molecular Polarity Table Molecular Geometry Molecular Shapes Molecular
https://i.pinimg.com/736x/4c/72/ed/4c72ed42a8b007fe71eb9201118abcc6--math-activities-chemistry.jpg

VSEPR Polarity And Bonds YouTube
https://i.ytimg.com/vi/40mG2rQlLpk/maxresdefault.jpg
What 5 molecular geometries are always polar 1 EG tetrahedral MG trigonal planar 2 EG tetrahedral MG bent 3 EG Trig Bipy MG seesaw 4 EG trig bipy MG T shape 5 EG May 7 2020 nbsp 0183 32 A polar molecule has an asymmetric shape lone electron pair or central atom bonded to other atoms with different electronegativity values Usually a polar molecule
Jul 16 2020 nbsp 0183 32 To determine if this molecule is polar we draw the molecular structure VSEPR theory predicts a linear molecule The C O bond is considerably polar Although C and S have very similar electronegativity Jan 21 2025 nbsp 0183 32 If there is only one bond in the molecule the bond polarity determines the molecular polarity Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule A diatomic molecule that
More picture related to What Molecular Shape Is Always Polar

Molecular Geometry Definition Chart Shapes And Examples
https://www.chemistrylearner.com/wp-content/uploads/2023/08/Molecular-Geometry-768x778.jpg
Solved A Molecule Has A Central Atom Surrounded By 1 lone Chegg
https://media.cheggcdn.com/study/6ab/6abc6ee2-5282-4020-b848-cef8a0190604/image

Polar Vs Nonpolar Bonds Overview Examples Expii Ionic Bonding
https://i.pinimg.com/originals/5b/90/f7/5b90f7c530e902342a30e2c5c0c1e7a2.jpg
A molecule can possess polar bonds and still be nonpolar If the polar bonds are evenly or symmetrically distributed the bond dipoles cancel and do not create a molecular dipole For example the three bonds in a molecule of BF 3 are Feb 14 2017 nbsp 0183 32 In simple terms polarity occurs when the electron distribution in a molecule is asymmetric This results in a net dipole moment in the molecule One end of the molecule is charged negative while the other gets a positive charge
Properties of Polar Molecules Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end Symmetry is the apt tool to decide whether a molecular shape is polar or not In general presence of spherical symmetry in a molecule renders it non polar and any sort of deviation from the
Solved A Molecule Has A Central Atom Surrounded By 1 lone Chegg
https://media.cheggcdn.com/study/75c/75c46204-501e-4e56-ac80-68c65261d06f/image
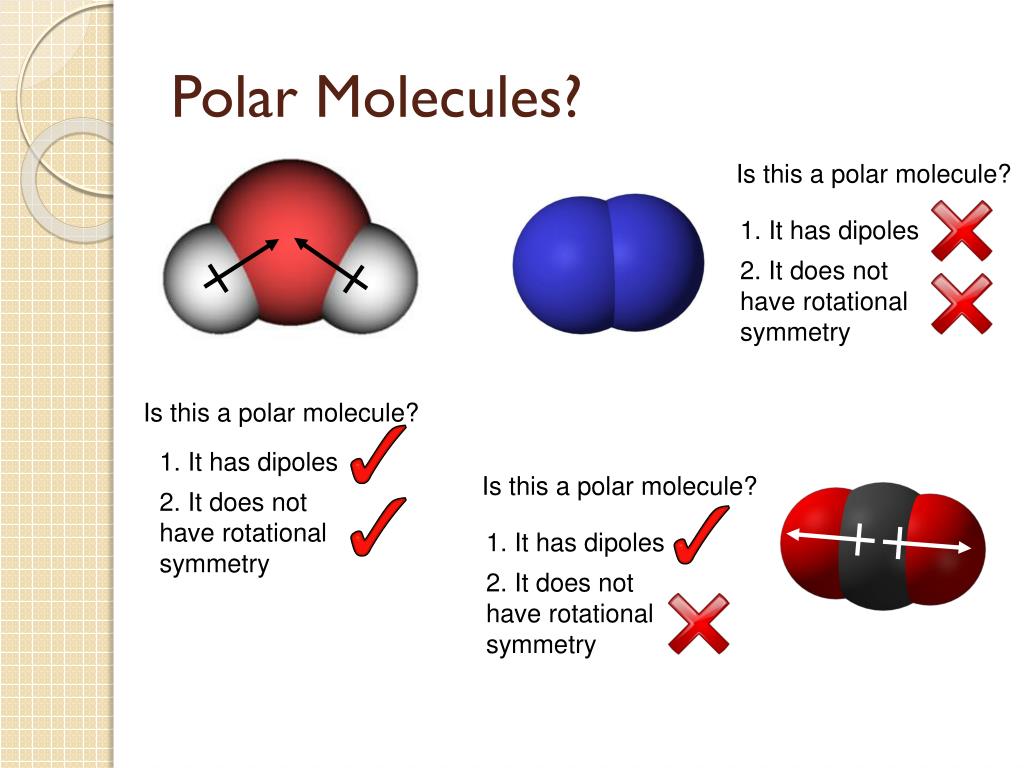
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint
https://image2.slideserve.com/4261659/polar-molecules1-l.jpg
What Molecular Shape Is Always Polar - The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms The molecular polarity can be established by determining

