What Isotope Has 18 Protons And 22 Neutrons 3 days ago nbsp 0183 32 An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior
Sep 13 2019 nbsp 0183 32 Get the definition of an isotope See examples of isotopes and learn the difference between an isotope and a nuclide of an element Feb 4 2020 nbsp 0183 32 Every element on the periodic table has multiple isotope forms The chemical properties of isotopes of a single element tend to be nearly identical the exceptions are the
What Isotope Has 18 Protons And 22 Neutrons

What Isotope Has 18 Protons And 22 Neutrons
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Fluorine.png

Isotope Notation Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/isotope_notation_calculations.jpeg
Understanding Protons Electrons And Neutrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d
Aug 19 2022 nbsp 0183 32 For example the naturally occurring isotope carbon 14 present in water is used to understand the age of water and other organic materials Read the IAEA Bulletins to learn An isotope is a variation of an element that possesses the same atomic number but a different mass number A group of isotopes of any element will always have the same number of
An isotope is named after the element and the mass number of its atoms For example carbon 12 is an isotope of carbon with a mass number of 12 All three isotopes of hydrogen have identical Jan 2 2013 nbsp 0183 32 According to the definition of isotope each of the same element has the same atomic number Z but each has a different mass number A The atomic number corresponds
More picture related to What Isotope Has 18 Protons And 22 Neutrons

Isotope Symbol For Ion
https://us-static.z-dn.net/files/dec/b65e03c11181ace194072418ff3351b9.png
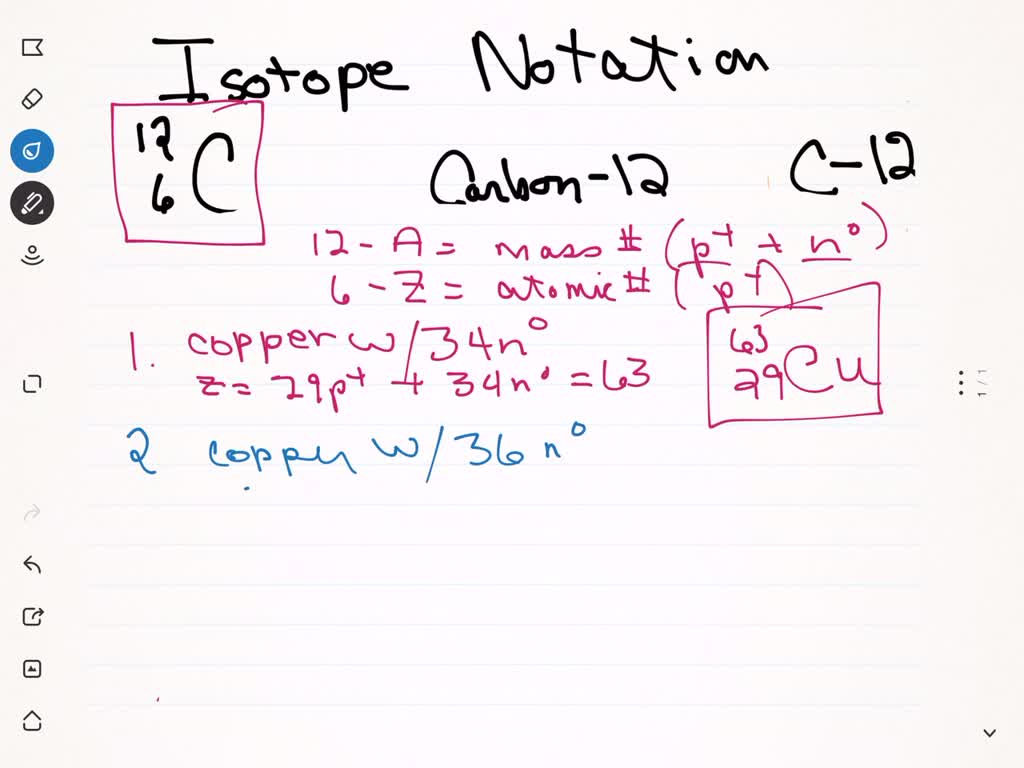
Write The Symbol For Each Isotope In The Form Azx Printable Form
https://cdn.numerade.com/previews/77372ed8-64c7-4811-91db-66d99d315491_large.jpg
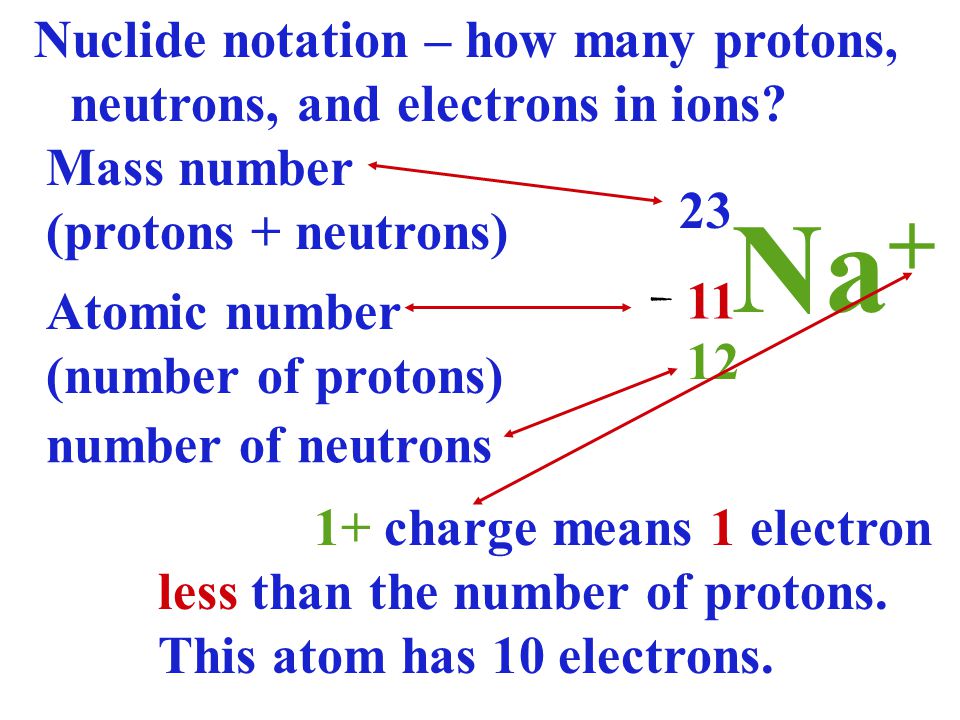
SimplyChemistry C1 1 2 PROTON NUMBER MASS NUMBER IONS ISOTOPES
https://3.bp.blogspot.com/-xjR7quI6CFs/VYLYdrkjBiI/AAAAAAAABeA/I1ufwOUUHhA/s1600/slide_9.jpg
In this concept tutorial learn about what an isotope is some common isotopes and their uses and how isotopes form and breakdown Summary Isotopes are atoms that have the same atomic number but different mass numbers due to a change in the number of neutrons The term nuclide refers to the nucleus of a given
[desc-10] [desc-11]

Electron Proton Neutron
https://d1avenlh0i1xmr.cloudfront.net/9608f5f5-e406-4832-bf83-648f0c2a0609/13.-aluminium-atom-teachoo-01.png
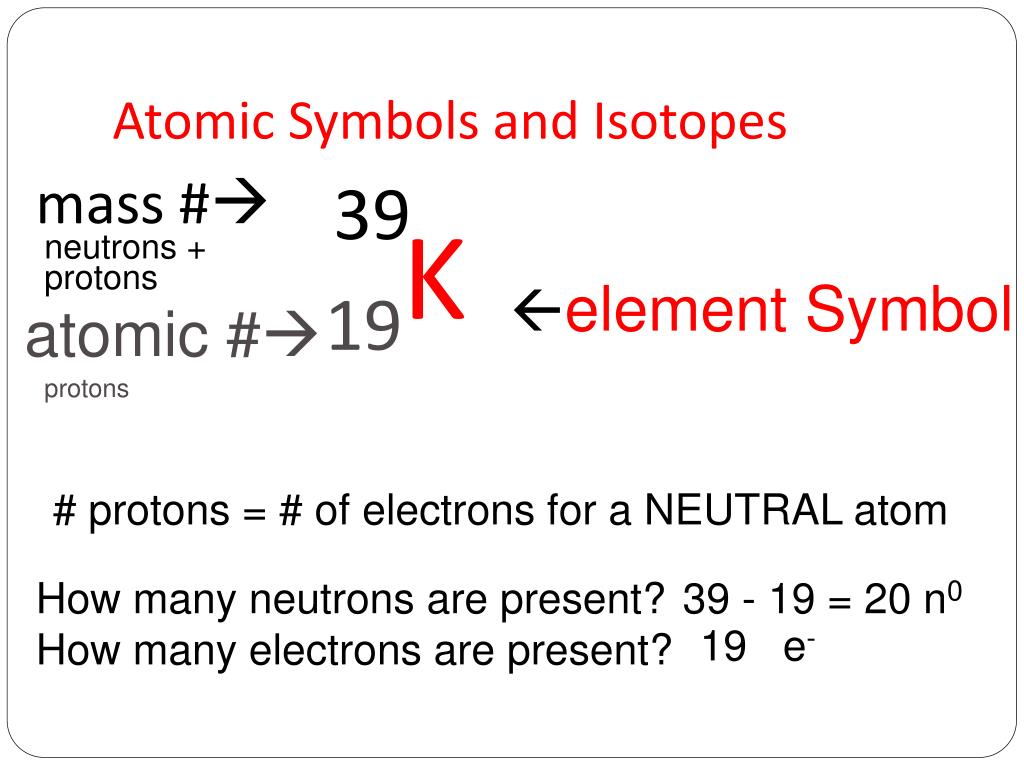
Isotope Symbol Electrons
https://image1.slideserve.com/3180031/atomic-symbols-and-isotopes-l.jpg
What Isotope Has 18 Protons And 22 Neutrons - An isotope is named after the element and the mass number of its atoms For example carbon 12 is an isotope of carbon with a mass number of 12 All three isotopes of hydrogen have identical