What Is The Trend For Ionization Energy Sep 16 2020 nbsp 0183 32 Ionization energy is the energy required to remove an electron from an atom It is a periodic table trend that increases moving across the table and decreases moving down it By
Ionization Energy Trend in the Periodic Table General periodic trends In a group while moving from top to bottom it decreases It increases from left to right across a period 1 Trends in Jun 30 2023 nbsp 0183 32 Major periodic trends include electronegativity ionization energy electron affinity atomic radius melting point and metallic character Periodic trends arising from the
What Is The Trend For Ionization Energy

What Is The Trend For Ionization Energy
https://sciencenotes.org/wp-content/uploads/2020/07/periodicity-chemistry-1024x683.jpg
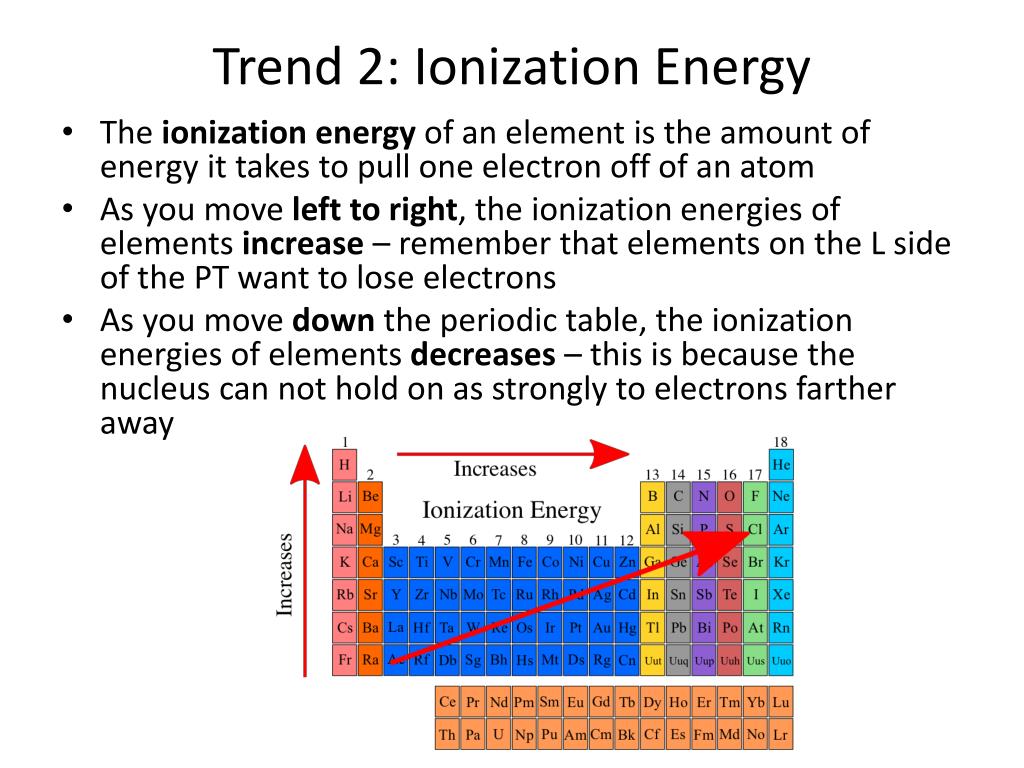
PPT Periodic Trends PowerPoint Presentation Free Download ID 3030966
https://image1.slideserve.com/3030966/trend-2-ionization-energy-l.jpg
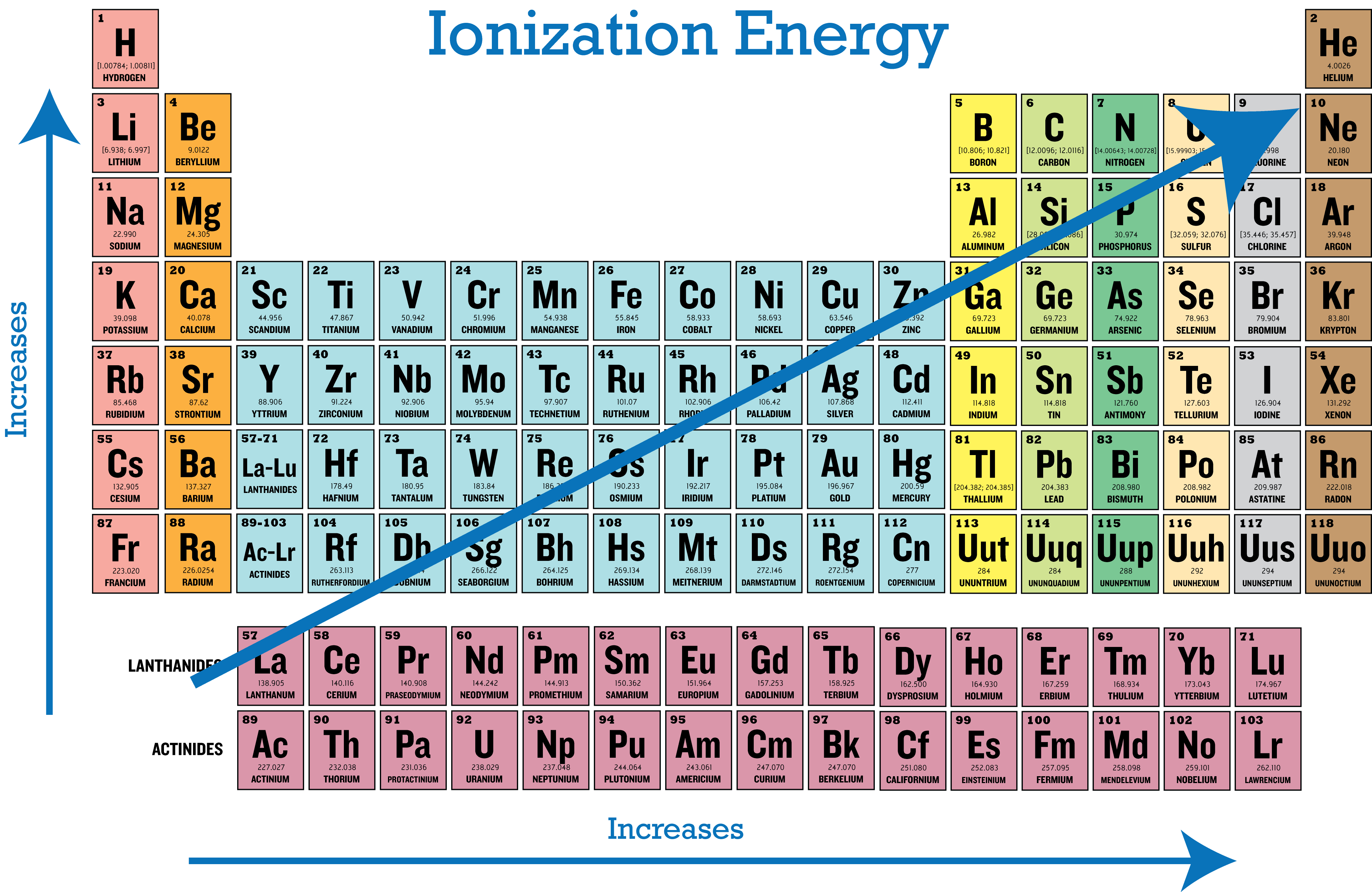
Untitled On Emaze
https://userscontent2.emaze.com/images/28c14443-ee6a-4c6c-bdac-74f4855f7f70/7fc737cffd8e17c7415158152d9727ec.png
Ionization energy is the minimum energy required to remove a loosely bound electron of an atom or molecule in the gaseous state It measures the capability of an atom to lose an electron What is ionization energy Learn the definition trend on the periodic table first amp second ionization energies see a chart and much more
Dec 27 2024 nbsp 0183 32 There are ionisation energy trends within periods and groups The ionisation energy increases as you remove more electrons from an element It is easy to remove Aug 12 2024 nbsp 0183 32 The general trend is for ionization energy to increase moving from left to right across an element period Moving left to right across a period the atomic radius decreases so
More picture related to What Is The Trend For Ionization Energy
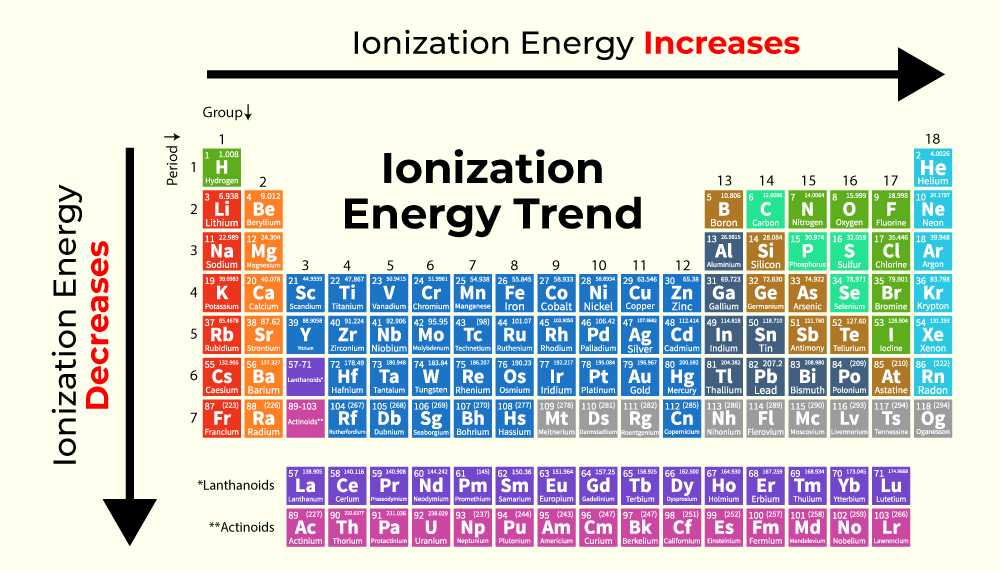
Ionization Energy Definition
https://media.geeksforgeeks.org/wp-content/uploads/20230310125901/ionization-energy-trend.png

Periodic Trends Part 2 Ionization Energy Electron Affinity
https://i.ytimg.com/vi/8JkpQ7J2g8c/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGBMgOCh_MA8=&rs=AOn4CLC0qCOo93VKCG3VO1i8a1qKKZEdBw

Ionisation Energy A Level Chemistry Revision Notes
https://alevelchemistry.co.uk/wp-content/uploads/2022/09/image-6.png
May 16 2023 nbsp 0183 32 It is the energy required to strip one or more electrons from a neutral atom to create a positively charged ion which alters the atom s chemical behavior The ionization Jul 25 2024 nbsp 0183 32 The trends in first ionization energy are shown in Figure PageIndex 1 and are summarized below Across a period As Z increases across a period the ionization energy of
[desc-10] [desc-11]

Electron Affinity Trend And Definition Electron Affinity Electrons
https://i.pinimg.com/originals/64/c1/0a/64c10a2710410e3c0fa7f4f83f4eacaf.png

Periodic Trend Electron Affinity Ionization Energy Modern Periodic
https://i.pinimg.com/originals/99/d6/6c/99d66c7846f4c733512c12a5402fdcc4.png
What Is The Trend For Ionization Energy - Ionization energy is the minimum energy required to remove a loosely bound electron of an atom or molecule in the gaseous state It measures the capability of an atom to lose an electron