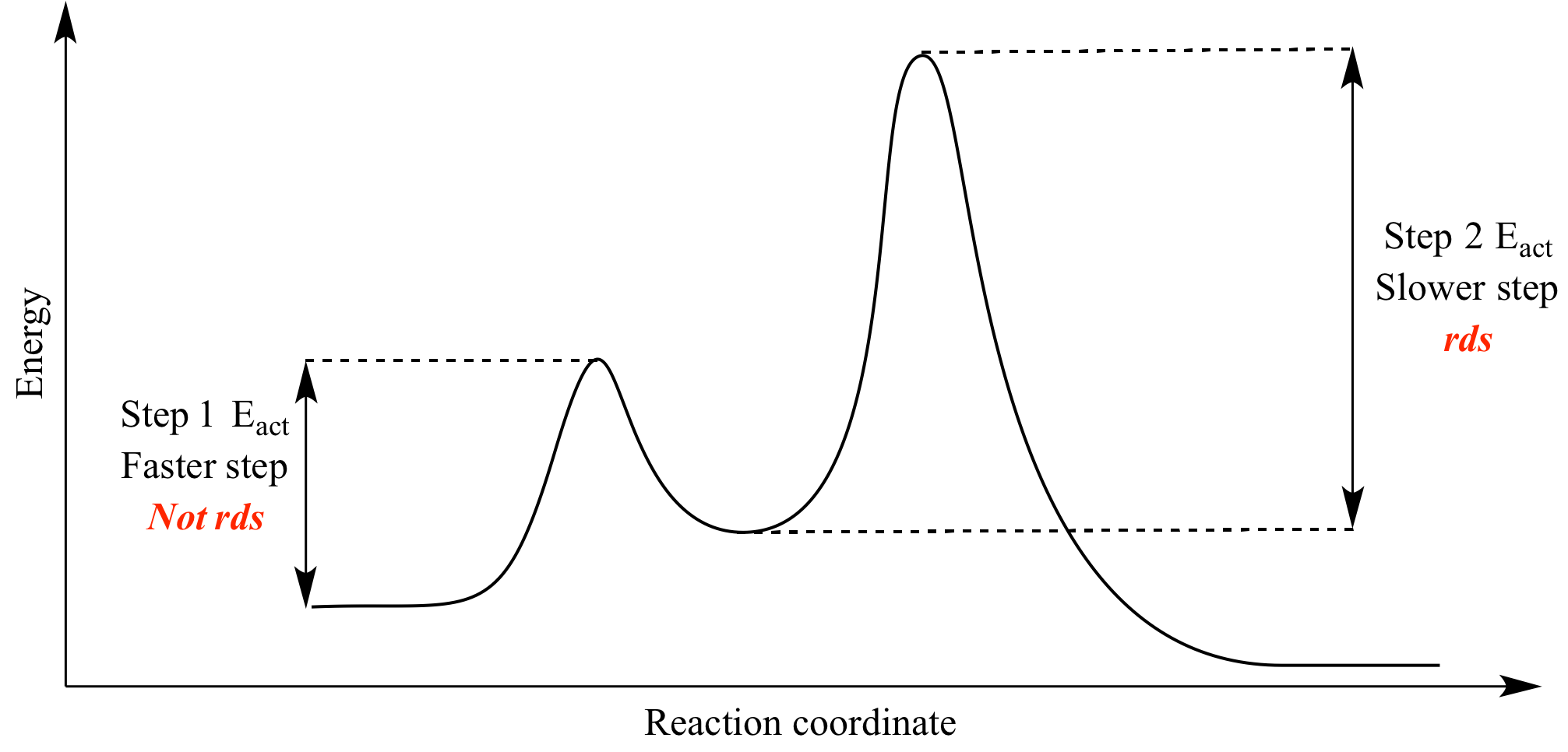
What Is The Rate Determining Step Of Michaelis Menten Kinetics Feb 20 2022 nbsp 0183 32 The correct answer is a The complex dissociation step to produce products For explanation I would say The breakdown of ES complex is the rate determining step of Michaelis Menten kinetics
In biochemistry Michaelis Menten kinetics named after Leonor Michaelis and Maud Menten is the simplest case of enzyme kinetics applied to enzyme catalysed reactions involving the transformation of one substrate into one product Jun 18 2022 nbsp 0183 32 The rate constants k 1 k 2 and k 3 describe the rates associated with each step of the catalytic process It is assumed that there is no significant rate for the backward reaction of enzyme and product E P being converted to ES complex
What Is The Rate Determining Step Of Michaelis Menten Kinetics
What Is The Rate Determining Step Of Michaelis Menten Kinetics
https://i.ytimg.com/vi/Va8OjE-wpXE/maxresdefault.jpg

Derivation Of Michaelis Menten Equation Part II YouTube
https://i.ytimg.com/vi/OOzj_dFzPH4/maxresdefault.jpg

Rate Determining Step Chemical Kinetics Chemistry Class 12 YouTube
https://i.ytimg.com/vi/uI2Ri4ONyh0/maxresdefault.jpg
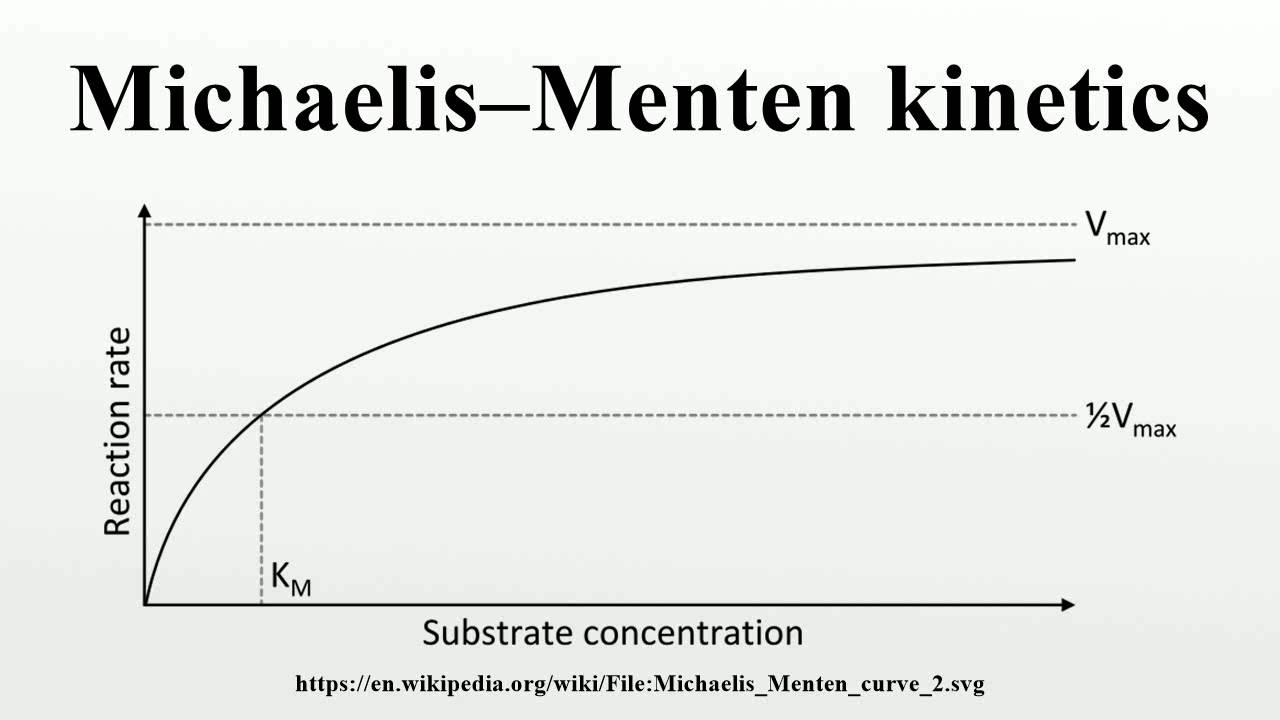
Apr 8 2024 nbsp 0183 32 Two important terms within Michaelis Menten kinetics are Vmax the maximum rate of the reaction when all the enzyme s active sites are saturated with substrate Km also known as the Michaelis constant the substrate concentration at which the The Michaelis Menten model for enzyme kinetics presumes a simple 2 step reaction Step 1 Binding the substrate binds to the enzyme Step 2 Catalysis the substrate is converted to product and released
It can be determined by taking the rate of step 2 which is a fundamental chemical process for which we can write the first order rate equation v o k 2 ES where ES is the concentration of enzyme actually occupied by substrate The simplified mechanism has two steps where the second step is rate determining The rate of the catalyzed reaction can be approximated as the rate law of step 2 However ES is a reactive intermediate whose concentration cannot be easily measured We need to express the reaction rate in terms of concentrations that are easily measurable
More picture related to What Is The Rate Determining Step Of Michaelis Menten Kinetics

Steady State Approximation Vs Rate Determining Step YouTube
https://i.ytimg.com/vi/p5PDmWDgWEo/maxresdefault.jpg

Determining Michaelis Menten Kinetics Parameters Using Nonlinear
https://i.ytimg.com/vi/EryNax2fJfY/maxresdefault.jpg
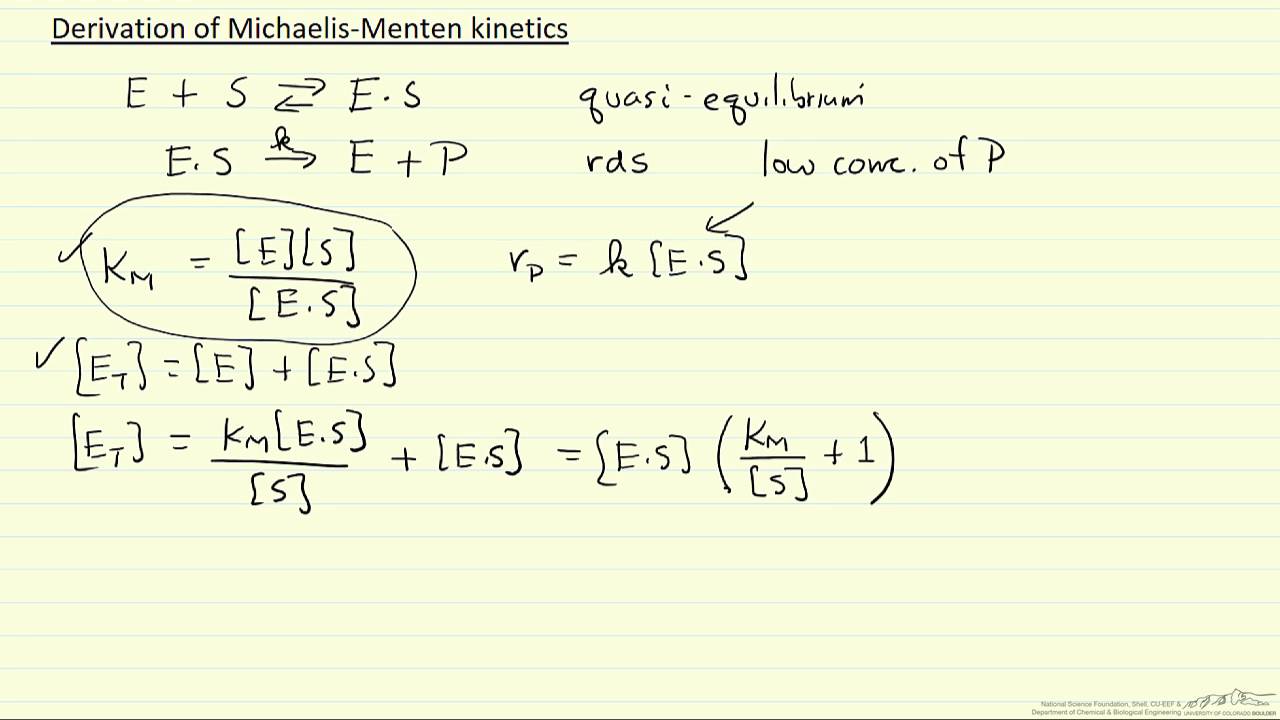
Derivation Of Michaelis Menten Kinetics YouTube
https://i.ytimg.com/vi/IdY3I6wVrJw/maxresdefault.jpg
Two 20th century scientists Leonor Michaelis and Maud Leonora Menten proposed the model known as Michaelis Menten Kinetics to account for enzymatic dynamics The model serves to explain how an enzyme can cause kinetic rate enhancement of a reaction and explains how reaction rates depends on the concentration of enzyme and substrate Delineate the steps and approximations in the Michaelis Menten mechanism for enzyme kinetics Express the rate of change of reactant substrate change in terms of Michaelis Menten parameters Use a Lineweaver Burk plot equation to fit Michaelis Menten parameters
In order to determine the rate of product formation d P dt kcat ES we need to rearrange the equation above to calculate ES We know that the free enzyme concentration E is equal to the total enzyme concentration ET minus ES Making these substitutions gives us Apr 28 2020 nbsp 0183 32 At some point in school or university most of us met the Michaelis Menten model of enzyme kinetics providing a simplified description of the dependence of the rate of an enzyme catalysed reaction on substrate concentration This post explores the assumptions and derivation of the model its caveats and how we can fit it to data

Michaelis Menten Lineweaver Burk Plots In Excel Calculate Vmax And
https://i.ytimg.com/vi/nGYGH3sm2B8/maxresdefault.jpg
Illustrated Glossary Of Organic Chemistry Rate Determing Step
https://www.chem.ucla.edu/~harding/IGOC/R/rate_determining_step01.png
What Is The Rate Determining Step Of Michaelis Menten Kinetics - The simplified mechanism has two steps where the second step is rate determining The rate of the catalyzed reaction can be approximated as the rate law of step 2 However ES is a reactive intermediate whose concentration cannot be easily measured We need to express the reaction rate in terms of concentrations that are easily measurable