What Is The Meaning Of The Rate Of Reaction Each reaction rate coefficient k has a temperature dependency which is usually given by the Arrhenius equation where A is the pre exponential factor or frequency factor exp is the exponential function
The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds It is expressed as the concentration of the reactants consumed or products formed per unit time 1 4 The general expression for the reaction Two ways to measure the volume of a gas produced in a reaction The rate of reaction can be analysed by plotting a graph of mass or volume of product formed against time The graph shows this for
What Is The Meaning Of The Rate Of Reaction

What Is The Meaning Of The Rate Of Reaction
http://meaningofname.co/image/meaning-of-name-susan.png

Josephine Meaning Of Name
https://meaningofname.co/image/meaning-of-name-josephine.png

Richard Meaning Of Name
http://meaningofname.co/image/meaning-of-name-richard-2.png
Nov 13 2022 nbsp 0183 32 Rate Laws and Reaction Order The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law For example the rate of the gas phase decomposition of dinitrogen pentoxide May 13 2021 nbsp 0183 32 The reaction rate calculated for the reaction A B using Equation ref Eq1 is different for each interval this is not true for every reaction as shown below A greater change
Reaction rate in chemistry the speed at which a chemical reaction proceeds It is often expressed in terms of either the concentration amount per unit volume of a product that is formed in a unit of time or the concentration of a reactant that is The rate of a chemical reaction is proportional to concentration of reactants present As reactants are used up during the process the rate will decrease and the reaction slows down
More picture related to What Is The Meaning Of The Rate Of Reaction

Lee Meaning Of Name
http://meaningofname.co/image/meaning-of-name-lee-1.png
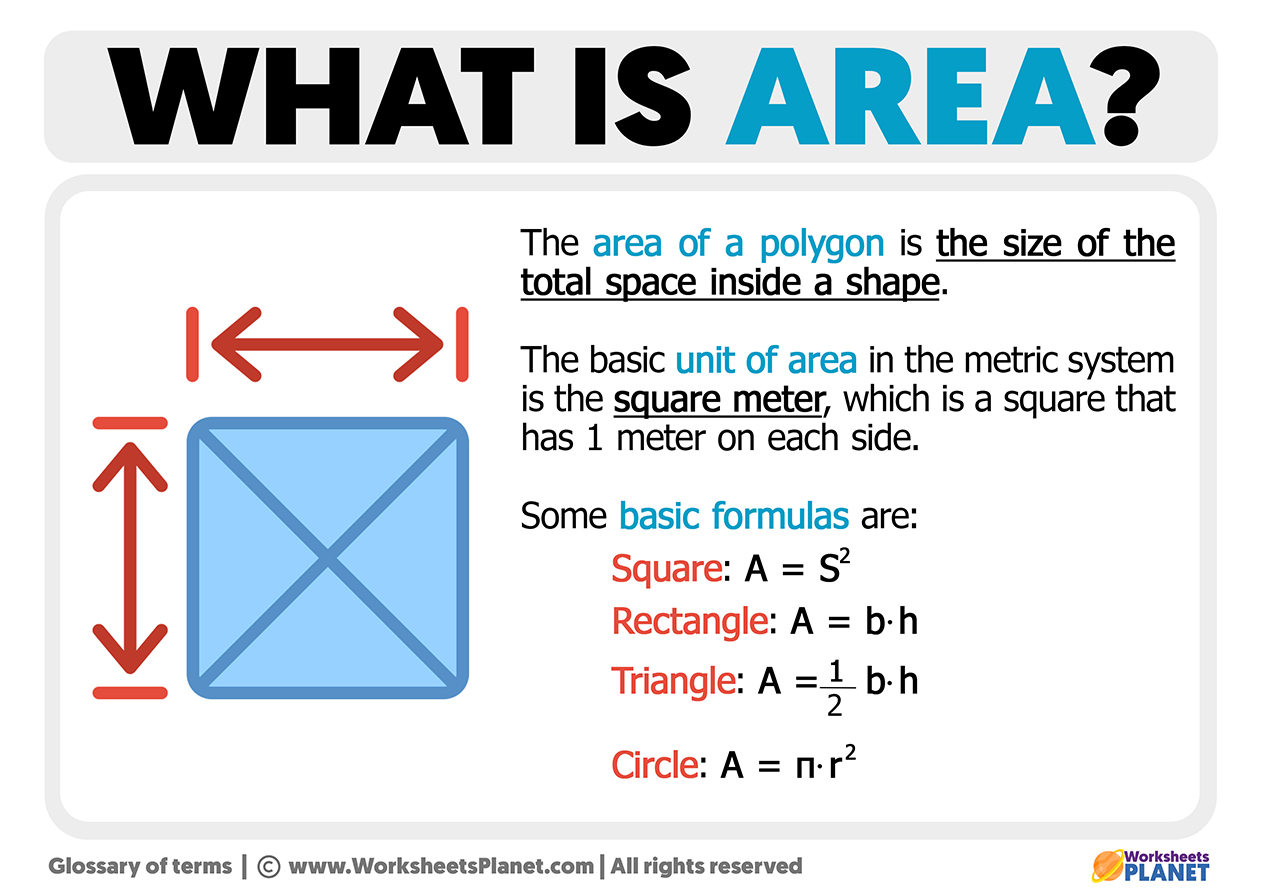
What Is Area
https://www.worksheetsplanet.com/wp-content/uploads/2022/12/What-is-area.jpg
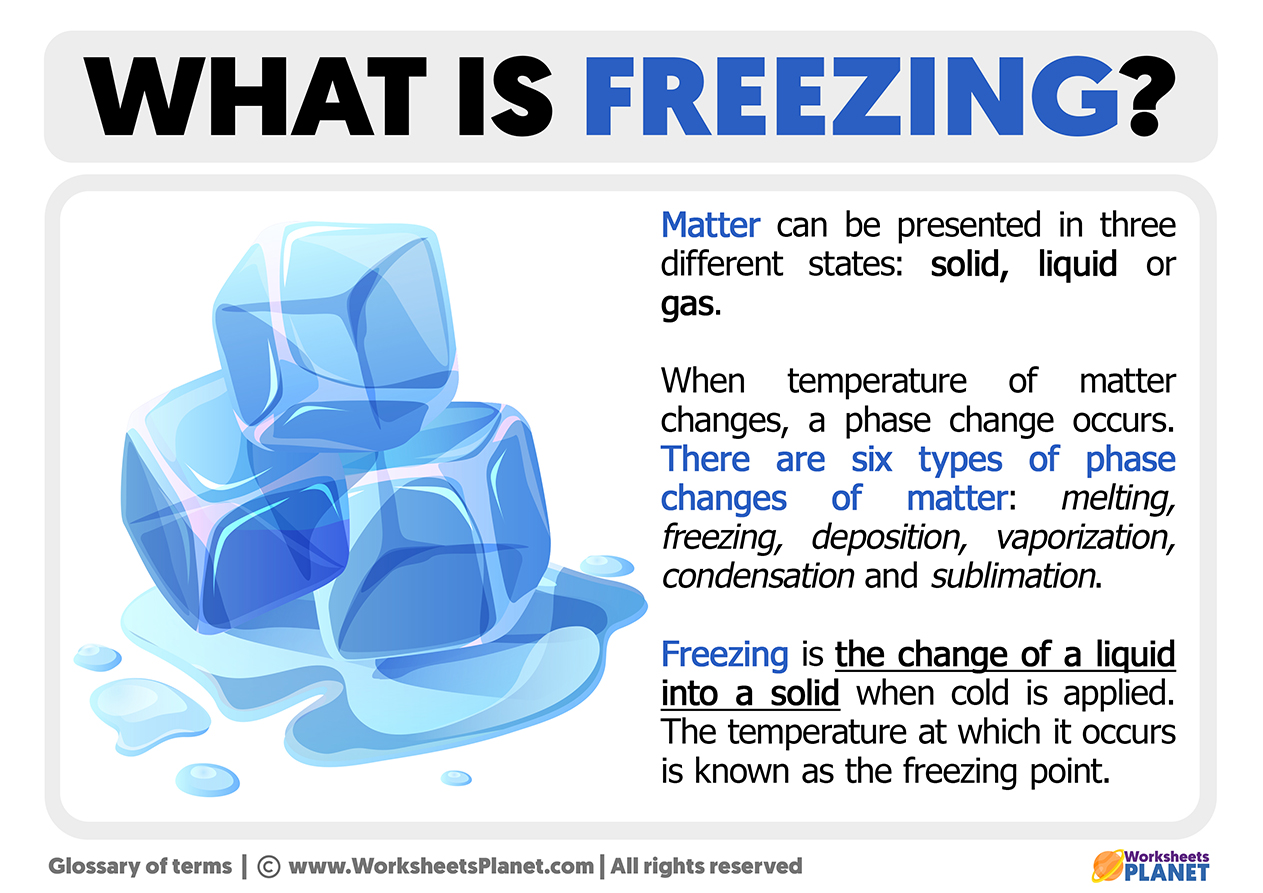
What Is Freezing
https://www.worksheetsplanet.com/wp-content/uploads/2022/12/What-is-freezing.jpg
Oct 1 2019 nbsp 0183 32 The reaction rate is defined as the rate at which the reactants of a chemical reaction form the products Reaction rates are expressed as concentration per unit time The rate of a chemical equation can be calculated Firstly it s important to understand what a rate of reaction is When a reaction occurs molecules are colliding together with enough energy for reactants to be broken down or changed into a
A reaction rate shows the rates of production of a chemical species It can also show the rate of consumption of a species for example a reactant In general though we want an overall Reaction rates are usually expressed as the concentration of reactant consumed or the concentration of product formed per unit time The units are thus moles per liter per unit time
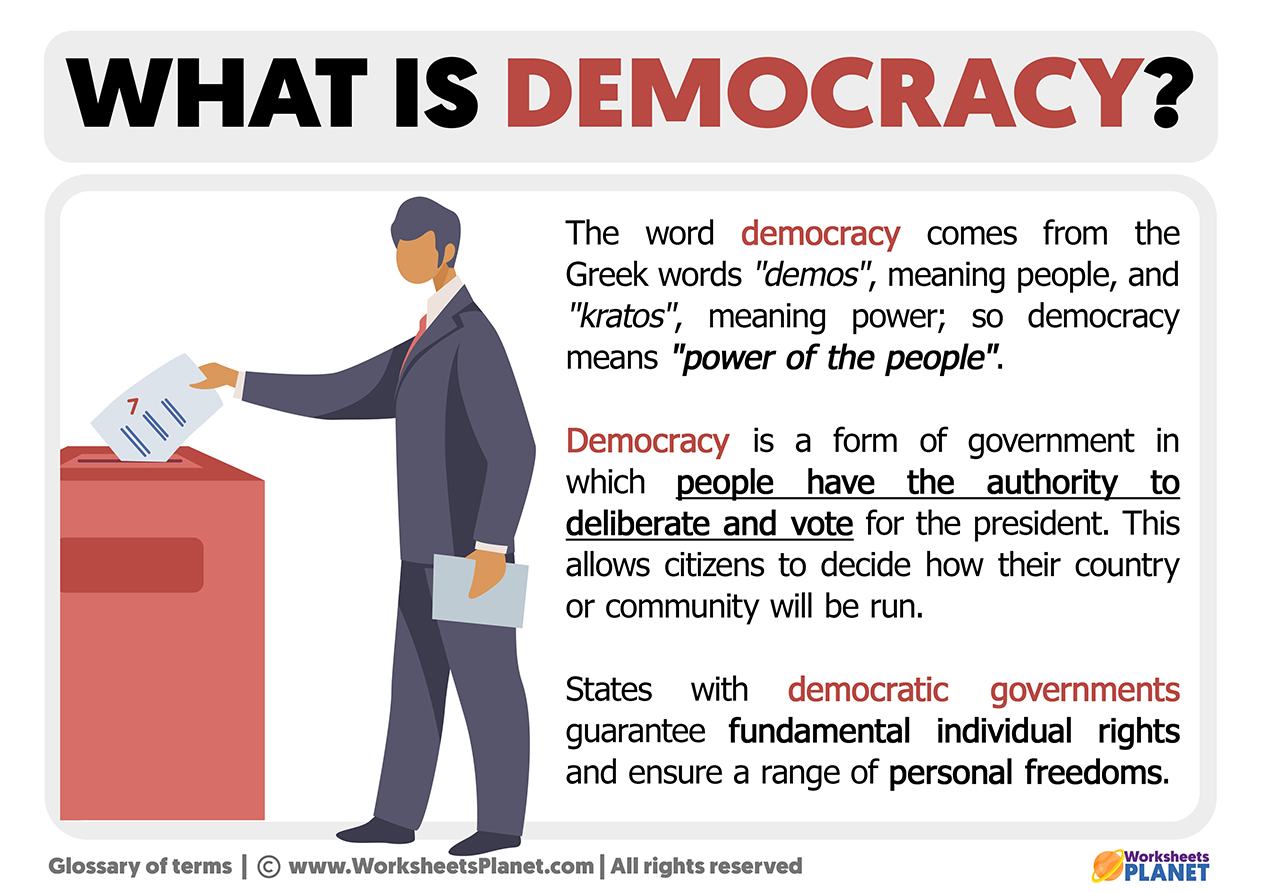
What Is Democracy
https://www.worksheetsplanet.com/wp-content/uploads/2022/10/What-is-Democracy.jpg

Question Video Determining The Unit Of Rate Of Reaction From The Units
https://media.nagwa.com/964198434874/en/thumbnail_l.jpeg
What Is The Meaning Of The Rate Of Reaction - Feb 13 2023 nbsp 0183 32 Definition of Reaction Rate The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the